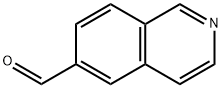
6-Isoquinolinecarboxaldehyde (9CI) synthesis
- Product Name:6-Isoquinolinecarboxaldehyde (9CI)
- CAS Number:173089-81-1
- Molecular formula:C10H7NO
- Molecular Weight:157.17

1105709-94-1
2 suppliers
inquiry

173089-81-1
52 suppliers
$45.00/25mg
Yield: 94%
Reaction Conditions:
Stage #1:6-vinylisoquinoline with ozone in methanol;dichloromethane at -78; for 0.25 h;
Stage #2: with dimethylsulfide;sodium hydrogencarbonate in methanol;dichloromethane at 20;
Steps:
6; 4
[00212] Isoquinoline-6-carbaldehyde: To a 150 mL round-bottomed flask was added 6-vinylisoquinoline (2.47 g, 16 mmol) and MeOH (35 mL)/DCM (35 mL). The resulting solution was cooled to -78°C. The reaction was ozonized until a blue color persisted, then nitrogen gas was bubbled through the solution for 15 minutes to purge the ozone. The reaction was then treated with solid sodium bicarbarbonate. (1.5 g) and dimethyl sulfide (3.2 mL, 0.2 mL/mmol of SM) and the mixture was warmed to room temperature and stirred overnight. The reaction mixture was diluted with water (100 mL)
References:
AMGEN INC. WO2009/11880, 2009, A2 Location in patent:Page/Page column 100-101
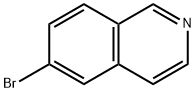
34784-05-9
317 suppliers
$5.00/1g
201230-82-2
1 suppliers
inquiry

173089-81-1
52 suppliers
$45.00/25mg
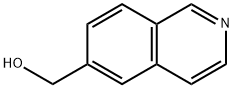
188861-59-8
42 suppliers
$17.00/50mg

173089-81-1
52 suppliers
$45.00/25mg

75476-84-5
34 suppliers
$145.00/100mg

173089-81-1
52 suppliers
$45.00/25mg
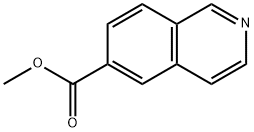
173089-82-2
59 suppliers
$98.00/100mg

173089-81-1
52 suppliers
$45.00/25mg