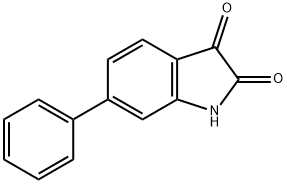
6-Phenylisatin synthesis
- Product Name:6-Phenylisatin
- CAS Number:109497-00-9
- Molecular formula:C14H9NO2
- Molecular Weight:223.23
Yield:109497-00-9 3.7%
Reaction Conditions:
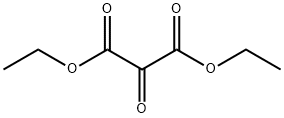
Stage #1: 3-biphenyl amine;Diethyl ketomalonatewith acetic acid at 110 - 120; for 8 h;
Stage #2: with potassium hydroxide at 120; pH=11; for 18 h;
Steps:
4.2. Synthesis of C5- and C6-substituted isatin analogues (9a-j)
General procedure: The C5- and C6-substituted isatin analogues (9a-j) investigated in this study were synthesized according to a modification of the literature description.23 A mixture of the appropriate C4- or C5-substituted aniline (10, 20 mmol) and acetic acid (35 mL) was heated at 110-120 °C to yield a clear solution. For this purpose either the free base or hydrochloride salt of the aniline may be used. Diethyl ketomalonate (20 mmol) was added to the reaction and the resulting solution was heated under reflux at 110-120 °C for 8 h. The residual acetic acid was removed by steam distillation and the pH of the reaction mixture was adjusted to 11 with potassium hydroxide (10%). The reaction was again heated under reflux at 120 °C for a period of 18 h while a stream of air was passed continuously through the reaction mixture. The reaction was cooled to room temperature and gravity filtered to yield a clear filtrate. The pH of the filtrate was adjusted to 3 with hydrochloric acid (20%) and the resulting precipitate was removed by vacuum filtration. The pH of the filtrate was adjusted to <1 with hydrochloric acid (20%) and the resulting orange to red precipitate was collected by vacuum filtration. The precipitate (dissolved in ethyl acetate) was applied to a short silica gel column (35 × 80 mm) and eluted with ethyl acetate as mobile phase. Elution of the target isatin analogues were monitored by silica gel TLC using ethyl acetate/petroleum ether (50:50) as mobile phase and the plates were visualised under UV light (254 nm). The products thus obtained were recrystallized from ethyl acetate. For previously described 9g and 9h the melting points were found to be 208-211 °C and 231-233 °C while the reported melting points are 208-210 °C and 235 °C, respectively.23 V.W. Langenbeck, K. Rühlmann, H.H. Reif and F. Stolze, J. Prakt. Chem. 4 (1957), p. 136.23
References:
Manley-King, Clarina I.;Bergh, Jacobus J.;Petzer, Jacobus P. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 1,p. 261 - 274] Location in patent:experimental part

63608-68-4
2 suppliers
inquiry

109497-00-9
8 suppliers
inquiry
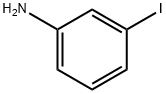
626-01-7
254 suppliers
$8.00/5g

109497-00-9
8 suppliers
inquiry
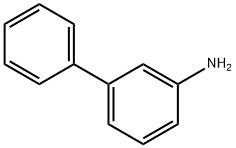
2243-47-2
217 suppliers
$6.00/1g

109497-00-9
8 suppliers
inquiry