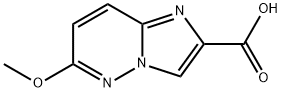
6-Methoxy-iMidazo[1,2-b]pyridazine-2-carboxylic acid synthesis
- Product Name:6-Methoxy-iMidazo[1,2-b]pyridazine-2-carboxylic acid
- CAS Number:64068-09-3
- Molecular formula:C8H7N3O3
- Molecular Weight:193.16
![ETHYL 6-CHLOROIMIDAZO[1,2-B]PYRIDAZINE-2-CARBOXYLATE](/CAS/GIF/64067-99-8.gif)
64067-99-8
132 suppliers
$8.00/100mg
![6-Methoxy-iMidazo[1,2-b]pyridazine-2-carboxylic acid](/CAS/20150408/GIF/64068-09-3.gif)
64068-09-3
19 suppliers
$278.00/100mg
Yield:64068-09-3 76%
Reaction Conditions:
with lithium hydroxide monohydrate in methanol;water at 100; for 0.25 h;Microwave irradiation;
Steps:
59A Intermediate 59A. Preparation of 6-methoxyimidazo[1,2-b]pyridazine-2-carboxylic acid
Combined ethyl 6-chloroimidazo[1,2-b]pyridazine-2-carboxylate (0.298 g, 1.321 mmol)iOH.H2O (2.64 mL, 2.64 mmol) in MeOH (4 mL)/H2O (4 mL) and heated to 100 °C inwave for 15 min. The solvents were reduced and remainder was acidified with 1 N HCl e resultant tan solid was collected by filtration to afford 6-methoxyimidazo[1,2-dazine-2-carboxylic acid (0.195 g, 1.01 mmol, 76 % yield). LCMS(ESI) m/z: 194.0 ]+ 1H NMR (400 MHz, DMSO-d6) δ 13.14 - 12.50 (m, 1H), 8.54 (d, J=0.7 Hz, 1H), 8.07=9.7, 0.7 Hz, 1H), 7.02 (d, J=9.7 Hz, 1H), 3.97 (s, 3H).
References:
WO2017/123860,2017,A1 Location in patent:Page/Page column 307; 308

64068-06-0
5 suppliers
inquiry
![6-Methoxy-iMidazo[1,2-b]pyridazine-2-carboxylic acid](/CAS/20150408/GIF/64068-09-3.gif)
64068-09-3
19 suppliers
$278.00/100mg
7252-84-8
213 suppliers
$9.00/250mg
![6-Methoxy-iMidazo[1,2-b]pyridazine-2-carboxylic acid](/CAS/20150408/GIF/64068-09-3.gif)
64068-09-3
19 suppliers
$278.00/100mg