
7 7 9 9-TETRAMETHYL-1 3 8-TRIAZASPIRO- synthesis
- Product Name:7 7 9 9-TETRAMETHYL-1 3 8-TRIAZASPIRO-
- CAS Number:15871-54-2
- Molecular formula:C11H19N3O2
- Molecular Weight:225.29
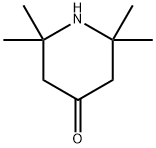
826-36-8
284 suppliers
$6.00/5g
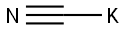
151-50-8
4 suppliers
$43.36/100g
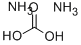
506-87-6
354 suppliers
$9.00/25g

15871-54-2
9 suppliers
inquiry
Yield:15871-54-2 91%
Reaction Conditions:
in ethanol;water at 85; for 12 h;
Steps:
1
In a Parr high-pressure reactor, 15.6 grams (0.1 mol) of 2,2, 6,6- tetramethyl-4-piperidone (Aldrich Chemicals Inc.), 13.5 grams (0.2 mol) of 97 % potassium cyanide, 43.2 grams (0.45 mol) of ammonium carbonate, 120 mL ethanol, and 120 mL water were mixed. The contents were reacted with stirring at 85°C for 12 hours, cooled to ambient temperature, and poured into 300 mL of water causing the solid product to precipitate. Impurity salts were washed away with water, and the product was dried. [00073] The product (white powder) was obtained in a 91% by weight yield. It had a decomposition point before melting at greater than 300°C. [00074] The product could be chlorinated as a suspension in aqueous 12 vol% sodium hypochlorite at about pH 7 (achieved by addition of HCI) to produce about 80% of the loaded oxidative chlorine theoretically expected as determined by iodometric/thiosulfate titration
References:
WO2005/58814,2005,A2 Location in patent:Page/Page column 23
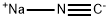
773837-37-9
0 suppliers
inquiry

826-36-8
284 suppliers
$6.00/5g

506-87-6
354 suppliers
$9.00/25g

15871-54-2
9 suppliers
inquiry