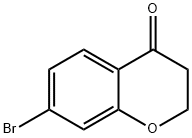
7-bromochroman-4-one synthesis
- Product Name:7-bromochroman-4-one
- CAS Number:18442-22-3
- Molecular formula:C9H7BrO2
- Molecular Weight:227.05

18386-03-3
21 suppliers
$148.00/100mg

18442-22-3
135 suppliers
$12.00/250mg
Yield:18442-22-3 85%
Reaction Conditions:
with acid activated montmorillonite K-10 in toluene; for 0.5 h;Reflux;Inert atmosphere;
Steps:
General experimental procedure for the synthesis of chroman-4-one (4a-4p)
General procedure: A mixture of 3-aryloxypropionic acid 3a-3o (1.0 mmol) and freshly prepared AA.Mont.K-10 (300% by weight) in toluene (2 mL) was heated to reflux under inert atmosphere for the specified time (30-45 minutes). After reaction completion, reaction mixture is cooled and added CH2Cl2 (10-15 mL). Filtered the AA.Mont.K-10 and washed twice with CH2Cl2. The organic filtrate is concentrated and the crude mass is extracted with hexane (10-15 mL) to afford pure chromanones 4a-4p.
References:
Begum, Ayisha F.;Balasubramanian, Kalpattu K.;Shanmugasundaram, Bhagavathy [Tetrahedron Letters,2021,vol. 82,art. no. 153372] Location in patent:supporting information
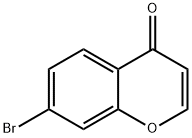
168759-60-2
54 suppliers
$31.00/200μmol

18442-22-3
135 suppliers
$12.00/250mg
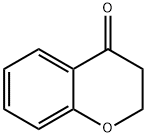
491-37-2
290 suppliers
$6.00/1g

18442-22-3
135 suppliers
$12.00/250mg
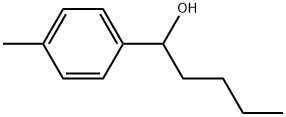
91763-35-8
1 suppliers
inquiry

18442-22-3
135 suppliers
$12.00/250mg
![Benzene, 1-bromo-3-[(3-chloro-2-propyn-1-yl)oxy]-](/CAS/20210305/GIF/890840-78-5.gif)
890840-78-5
0 suppliers
inquiry

18442-22-3
135 suppliers
$12.00/250mg