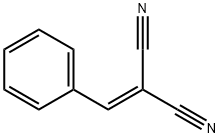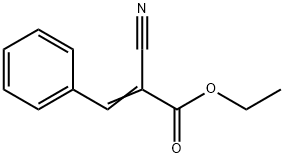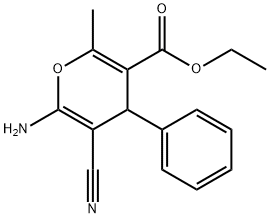
ETHYL 6-AMINO-5-CYANO-2-METHYL-4-PHENYL-4H-PYRAN-3-CARBOXYLATE synthesis
- Product Name:ETHYL 6-AMINO-5-CYANO-2-METHYL-4-PHENYL-4H-PYRAN-3-CARBOXYLATE
- CAS Number:72568-47-9
- Molecular formula:C16H16N2O3
- Molecular Weight:284.31

141-97-9
771 suppliers
$5.00/25g

100-52-7
947 suppliers
$20.37/250g

109-77-3
422 suppliers
$10.00/5g

72568-47-9
14 suppliers
inquiry
Yield:72568-47-9 100%
Reaction Conditions:
with 6C4H12N(1+)*6H2O*Nb10O28(6-) in neat (no solvent) at 80; for 1 h;Microwave irradiation;
Steps:
General procedure for the synthesis of 4H-pyrans
General procedure: The 1,3-Diketo compound, malononitrile and aromatic aldehydes were used in the synthesis of 4H-pyrans. All chemicals were purchased from Aldrich and used without further purification. A mixture of ethyl acetoacetate or methyl acetoacetate(1 mmol), aldehyde (1 mmol), and malononitrile (1 mmol), and the catalysts(100 mg) were placed in a microwave tube containing a magnetic stirrer under solvent-free conditions. This was heated under microwave irradiation at 80°C. The equipment used was an Anton Paar Monowave 400.The reaction progress was monitored by TLC (1:2 EtOAc: petroleum ether asmobile phase). The catalyst was recovered by filtration after precipitation of decaniobate in acetone (2 9 1 mL). The crude product was recrystallized using hot ethanol and no further recrystallization was required. The yield was expressed as the ratio of moles of products/product to moles of initial aldehyde (Table 1).
References:
Gutierrez, Luisa F.;Nope, Eliana;Rojas, Hugo A.;Cubillos, Jairo A.;Sathicq, ángel G.;Romanelli, Gustavo P.;Martínez, José J. [Research on Chemical Intermediates,2018,vol. 44,# 9,p. 5559 - 5568]

141-97-9
771 suppliers
$5.00/25g

89809-89-2
20 suppliers
$30.00/20mg

72568-47-9
14 suppliers
inquiry

100-52-7
947 suppliers
$20.37/250g
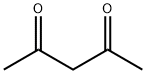
123-54-6
560 suppliers
$10.00/25ml

109-77-3
422 suppliers
$10.00/5g

72568-47-9
14 suppliers
inquiry