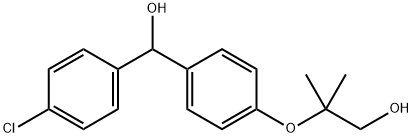
Benzenemethanol, 4-chloro-α-[4-(2-hydroxy-1,1-dimethylethoxy)phenyl]- synthesis
- Product Name:Benzenemethanol, 4-chloro-α-[4-(2-hydroxy-1,1-dimethylethoxy)phenyl]-
- CAS Number:72577-81-2
- Molecular formula:C17H19ClO3
- Molecular Weight:306.79
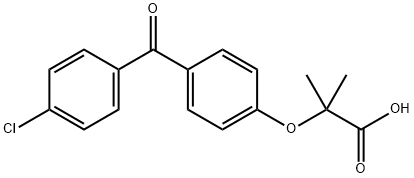
42017-89-0
386 suppliers
$5.00/100mg
![Benzenemethanol, 4-chloro-α-[4-(2-hydroxy-1,1-dimethylethoxy)phenyl]-](/CAS/20211123/GIF/72577-81-2.gif)
72577-81-2
0 suppliers
inquiry
Yield:72577-81-2 94%
Reaction Conditions:
Stage #1: fenofibric acidwith borane-THF in tetrahydrofuran at 0 - 50; for 3 h;Inert atmosphere;
Stage #2: with methanol in tetrahydrofuran at 0;
Steps:
12.C
Intermediate C: 2-(4-((4-Chlorophenyl)(hydroxy)methyl)phenoxy)-2-methylpropan-1 -olTo a stirred solution of intermediate B (500 mg, 1 .57 mmol) in dry THF (10 mL) at 0 °C under nitrogen was added a solution of borane in THF (1 M, 4.7 mL, 4.7 mmol) dropwise. The resulting mixture was heated at 50 °C for 3 h then cooled to 0 °C, quenched with MeOH and extracted with EtOAc (10 mL x 3). The combined organic layers were washed with brine, dried over MgS04 and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (Pet. ether/EtOAc, 5/1 to 2/1 , v/v) to give 2-(4-((4- chlorophenyl)(hydroxy)methyl)phenoxy)-2-methylpropan-1 -ol (452 mg, 94%) as a white solid.LC-MS (Agilent): Rt 3.00 min; m/z calculated for Ci7H19CI03 [M+Na]+ 329.1 , found 329.0.
References:
WO2012/63085,2012,A2 Location in patent:Page/Page column 84
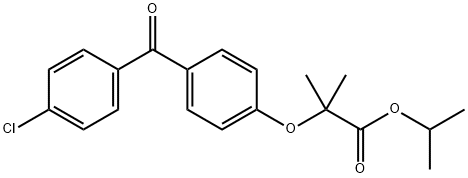
49562-28-9
618 suppliers
$5.00/250mg
![Benzenemethanol, 4-chloro-α-[4-(2-hydroxy-1,1-dimethylethoxy)phenyl]-](/CAS/20211123/GIF/72577-81-2.gif)
72577-81-2
0 suppliers
inquiry