
1-(2-HYDROXYPHENYL)-3-(4-ISOPROPYLPHENYL)PROP-2-EN-1-ONE synthesis
- Product Name:1-(2-HYDROXYPHENYL)-3-(4-ISOPROPYLPHENYL)PROP-2-EN-1-ONE
- CAS Number:73110-49-3
- Molecular formula:C18H18O2
- Molecular Weight:266.33
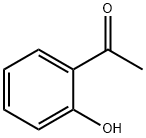
118-93-4
548 suppliers
$10.00/25g
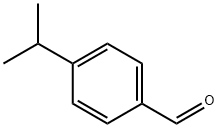
122-03-2
324 suppliers
$6.00/25g

73110-49-3
13 suppliers
$28.60/50MG
Yield:-
Reaction Conditions:
with sodium hydroxide in methanol; for 3 h;Reflux;Claisen-Schmidt Condensation;
Steps:
General procedure for 3-hydroxyflavone syntheses
General procedure: In a round-bottom flask (25 mL), equipped with magnetic stirrer 2-hydroxyacetophenone (a) (0.2 mL, 1.66 mmol), cumanaldehyde (b) (0.24 g, 1.66 mmol), sodium hydroxide (0.2 g, 5 mmol) and methanol(10 mL) were introduced. The pale yellow mixture was refluxed untilthe color was changed into orange (about 3 h). The reaction mixturewas cooled to room temperature and sodium hydroxide (0.5 N, 10 mL)and then hydrogen peroxide (35%, 0.684 mL) were added. After stirring2-3 h, the mixture was poured into ice-water and the formed precipitatewas filtered to obtain 1 as a yellow solid (75%) [22].The following were similarly prepared 2-46.4.2.1. 3-hydroxy-2-(4-isopropylphenyl)-4H-chromen-4-one (1) Yellow solid, yield: 75%, mp: 238 °C; 1H NMR (300 MHz, DMSO-d6)δH: 8.27 (2H, d, J=8.1 Hz, H-2′, H-6′), 8.12 (1H, dd, J1=7.5,J2=1.2 Hz, H-5), 7.77 (2H, m, H-6, H-7), 7.46-7.42 (3H, m, H-8, H-3′,H-5′), 2.92 (1H, sept, J=6.9 Hz, H-7′), 1.27 (6H, d, J=6.9 Hz, H-8′,H-9′). 13C NMR (75 MHz, DMSO-d6) δC: 175.1 (C-4), 154.7 (C-9), 149.9(C-4′), 145.5 (C-2), 133.4 (C-3), 130.3 (C-7), 129.6 (C-1′), 127.6 (C-2′,C-6′), 126.7 (C-3′, C-5′), 125.4 (C-5), 124.4 (C-6), 121.7 (C-10), 116.2(C-8), 33.8 (C-7′), 24.1 (C-8′, C-9′). DCI- HRMS [M+H]+ calcd. for(C18H17O3)+: 281.1178, found: 281.1183.
References:
Znati, Mansour;Bordes, Claire;Forquet, Valérian;Lantéri, Pierre;Ben Jannet, Hichem;Bouajila, Jalloul [Bioorganic Chemistry,2019,vol. 89,art. no. 103009]