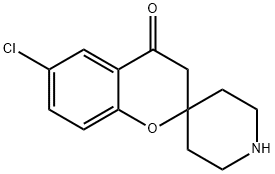
SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDIN]-4(3H)-ONE, 6-CHLORO- synthesis
- Product Name:SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDIN]-4(3H)-ONE, 6-CHLORO-
- CAS Number:792895-79-5
- Molecular formula:C13H14ClNO2
- Molecular Weight:251.71
![TERT-BUTYL 6-CHLORO-4-OXOSPIRO[CHROMAN-2,4'-PIPERIDINE]-1'-CARBOXYLATE](/CAS/GIF/1011482-37-3.gif)
1011482-37-3
17 suppliers
inquiry
![SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDIN]-4(3H)-ONE, 6-CHLORO-](/CAS/GIF/792895-79-5.gif)
792895-79-5
10 suppliers
inquiry
Yield:792895-79-5 88%
Reaction Conditions:
Stage #1: tert-butyl-6-chloro-4-oxospiro[chromanone-2,4'-piperidine]-1'-carboxylatewith trifluoroacetic acid in dichloromethane; for 24 h;
Stage #2: with sodium hydroxide in chloroform;water; pH=~ 12;
Steps:
23
A solution of 1-(5-chloro-2-hydroxyphenyl)ethanone (ASDI Inc., Newark, DE) (103.9 g, 0.609 mol), tert-butyl 4-oxopiperidine-1-carboxylate (2; 121.2 g, 0.609 mol) and pyrrolidine (50.8 mL, 0.609 mol) in methanol (500 mL) was stirred for 24 h, and then the precipitated crystals were separated by filtration and washed with hexane/ethyl acetate (9:1) mixture. Then hexane (150 mL) was added, and the obtained mixture was refluxed and then filtered. The separated crystals were dried to give N-Boc-6-chlorospiro- [chromene-2,4'-piperidin]-4(3H)-one in 72% (153 g) yield.
References:
WO2008/65508,2008,A1 Location in patent:Page/Page column 22-23
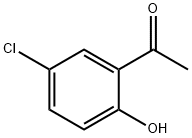
1450-74-4
255 suppliers
$8.00/5g
![SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDIN]-4(3H)-ONE, 6-CHLORO-](/CAS/GIF/792895-79-5.gif)
792895-79-5
10 suppliers
inquiry

79099-07-3
546 suppliers
$5.00/5g
![SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDIN]-4(3H)-ONE, 6-CHLORO-](/CAS/GIF/792895-79-5.gif)
792895-79-5
10 suppliers
inquiry