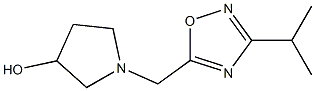
3-Pyrrolidinol, 1-[[3-(1-methylethyl)-1,2,4-oxadiazol-5-yl]methyl]- synthesis
- Product Name:3-Pyrrolidinol, 1-[[3-(1-methylethyl)-1,2,4-oxadiazol-5-yl]methyl]-
- CAS Number:832715-05-6
- Molecular formula:C10H17N3O2
- Molecular Weight:211.2609
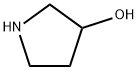
40499-83-0
202 suppliers
$14.00/1g
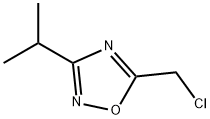
54042-97-6
37 suppliers
$60.00/100mg
![3-Pyrrolidinol, 1-[[3-(1-methylethyl)-1,2,4-oxadiazol-5-yl]methyl]-](/CAS/20180527/GIF/832715-05-6.gif)
832715-05-6
0 suppliers
inquiry
Yield:832715-05-6 82%
Reaction Conditions:
with potassium carbonate in acetonitrile at 65; for 1 h;
Steps:
9.65.1 Example 9. 65 : Preparation of 1-[2-Fluoro-4-(methanesulfonyl)phenyl]-4-[[1-(3-isopropyl-1, 2, 4- OXADIAZOL-5-YL)-3-PYRROLIDINYL] OXY]-1H-PYRAZOLO- [3, 4-DLPYRIMIDINE HYDROCHLORIDE (Compound A136); Step 1 : Preparation of (RS)-3-HYDROXY-1-1 (3-ISOPROPYL-1, 2, 4-oxadiazol-5- yl) methyl] pyrrolidine
A mixture OF 5-CHLOROMETHYL-3-ISOPROPYL- [1, 2, 4] OXADIAZOLE (1. 6 g, 10 mmol) and (RS)-3- hydroxypyrrolidine (960 mg, 11 mmol) were combined neat, diluted with MECN (10 mL), and K2CO3 (2. 75 g, 20 mmol) was added. The mixture was heated at 65°C for 1 h, and was filtered upon cooling. The solvent was removed and the residue was taken up in CH2C12 and rinsed with water. The organic extract was dried over MGSO4, the solvent was removed, and the residue was taken up in ether and filtered in order to remove a small amount of quaternary ammonium byproduct. Solvent removal from the filtrate gave (RS)-3-HYDROXY-1- [ (3-ISOPROPYL-1, 2, 4-OXADIAZOL-5-YL) methyl] pyrrolidine as an amber oil (1. 72 g, 82% yield) :'H NMR (DMSO-D6) 5 4. 76 (d, 1 H, J= 4. 5 Hz), 4. 19 (M, 1 H), 3. 90 (S, 2 H), 3. 05 (M, 1 H), 2. 79 (M, 1 H), 2. 69 (M, 1 H), 2. 53 (M, 1 H), 2. 43 (M, 1 H), 1. 98 (M, 1 H), 1. 54 (M, 1 H), 1. 25 (d, 6 H, J= 6. 8 Hz) ; MS mlz 212. 1 (M+).
References:
WO2005/7658,2005,A2 Location in patent:Page 214