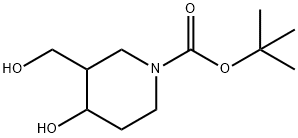
tert-butyl 4-hydroxy-3-(hydroxymethyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-hydroxy-3-(hydroxymethyl)piperidine-1-carboxylate
- CAS Number:849767-19-7
- Molecular formula:C11H21NO4
- Molecular Weight:231.29
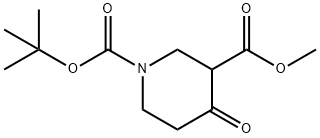
161491-24-3
171 suppliers
$8.00/1g

849767-19-7
15 suppliers
$65.00/10mg
Yield:849767-19-7 1900 mg
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0 - 60; for 2 h;
Steps:
130 Preparation of ferf-butyl 4-hydroxy-3-(hydroxymethyl)piperidine-l-carboxylate
To a stirred solution of 1 -teri-butyl 3-methyl 4-oxopiperidine-l,3-dicarboxylate (2350 mg; 8.86 mmol)[l 61491-24-3] in methanol (50 mL) was added sodium borohydride (1030 mg; 26.6 mmol) at 0 °C. The reaction mixture was then heated to 60 °C and stirred for 2 hours. After cooling to room temperature, the reaction mixture was treated with IN HC1 aqueous solution (10 mL) and the product was extracted with diethyl ether (4 x 40 mL). The combined organic layers were dried over Na2S04, filtered and concentrated to dryness. The residue was purified by column chromatography (silica gel; petroleum ether: acetone; 3:2; v/v) to afford feri-butyl 4-hydroxy-3-(hydroxymethyl)piperidine-l-carboxylate (1900 mg) as a yellow oil.
References:
WO2018/2220,2018,A1 Location in patent:Page/Page column 132-133
98977-34-5
204 suppliers
$5.00/250mg

849767-19-7
15 suppliers
$65.00/10mg
71486-53-8
144 suppliers
$6.00/1g

849767-19-7
15 suppliers
$65.00/10mg

24424-99-5
817 suppliers
$13.50/25G

849767-19-7
15 suppliers
$65.00/10mg
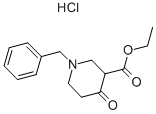
1454-53-1
237 suppliers
$5.00/5g

849767-19-7
15 suppliers
$65.00/10mg