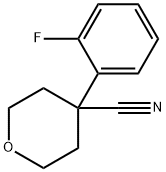
4-(2-Fluorophenyl)tetrahydropyran-4-carbonitrile, 97+% synthesis
- Product Name:4-(2-Fluorophenyl)tetrahydropyran-4-carbonitrile, 97+%
- CAS Number:859164-45-7
- Molecular formula:C12H12FNO
- Molecular Weight:205.23
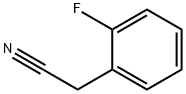
326-62-5
252 suppliers
$5.00/5g

859164-45-7
20 suppliers
$41.00/250mg
Yield:859164-45-7 80%
Reaction Conditions:
with sodium hydride in dimethyl sulfoxide at 20; for 0.5 h;
Steps:
22
Preparation 22; 4- (2-Fluoro-phen)-tetrahydro-pyran-4-carbonitrile ;Slowly add NaH (920mg, 23. 0mmol) to a solution of (2-fluorophenyl)-acetonitrile (1.27mL, 9.95mmol) in DMSO (40. 0mL). Stir the mixture at RT for 30mins then add via cannula a solution of 1,3-dichloropropane (l. OmL, 8. 53mmol) in DMSO (20mL). After addition is complete stir at 75°C for 5h. Pour mixture over ice (60g) and extract with Et2O (3 X 50mL). Combine the organic solutions and wash with brine (50mL), then dry filter and concentrate. Purify the material by flash chromatography (using a linear gradient of 100% hexanes to 35% EtOAc/hexanes) to give the title compound (1.4g, 80%) as a yellow oil. 1H NMR (400MHz, CDC13) : 8 7.43 (dt, 1H, J = 1.7, 7.9), 7.36 (m, 1H), 7.19 (dt, 1H, J = 1. 4,7. 7), 7.13 (ddd, 1H, J = 1.4, 6.6, 14.5), 4.08 (m, 2H), 3.94 (dt, 2H, J = 1. 7,7. 9), 2.26 (dt, 2H, J = 4. 4,13. 7), 2.19 (m, 2H).
References:
WO2005/66126,2005,A1 Location in patent:Page/Page column 43-44

326-62-5
252 suppliers
$5.00/5g
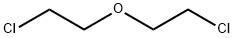
111-44-4
314 suppliers
$13.00/50g

859164-45-7
20 suppliers
$41.00/250mg