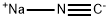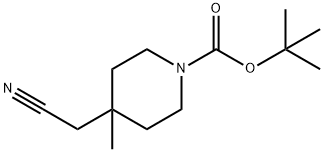
tert-butyl 4-(cyanomethyl)-4-methylpiperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(cyanomethyl)-4-methylpiperidine-1-carboxylate
- CAS Number:872850-30-1
- Molecular formula:C13H22N2O2
- Molecular Weight:238.33
872850-29-8
7 suppliers
inquiry

872850-30-1
15 suppliers
inquiry
Yield:872850-30-1 86%
Reaction Conditions:
with water;lithium chloride in dimethyl sulfoxide at 160; for 2.5 h;
Steps:
H.3
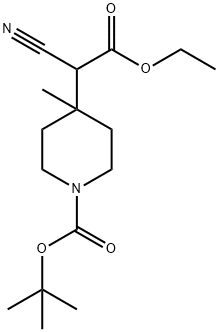
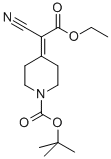
Step 3: Preparation of tert-buty 4-(cyanomethyl)-4-methylpiperidine-l-carboxylate; CNTo a solution of tert-butyl 4-(l-cyano-2-ethoxy-2-oxoethyl)-4-methylpiperidine-l-carboxylate (13. 3g, 42.8mmol) in DMSO (120ml) and water (1.5ml) was added lithiumchloride (2.54g) and the resulting mixture was heated to 160°C for 2.5 hours. The reactionwas cooled to room temperature and water (200ml) was added. The mixture was extractedwith diethyl ether (800ml), washed with brine and dried. Evaporation under reduced pressureyielded a tan solid (8.77g, 86%); NMR (CDC13): 1.1 (s, 3H), 1.4 (m, 13H), 2.2 (s, 2H), 3.1-3.2(m, 2H), 3.5 (m, 2H).
References:
WO2006/1752,2006,A1 Location in patent:Page/Page column 72

79099-07-3
543 suppliers
$5.00/5g

872850-30-1
15 suppliers
inquiry
193537-11-0
30 suppliers
inquiry

872850-30-1
15 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-methyl-4-[[(methylsulfonyl)oxy]methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/614730-89-1.gif)