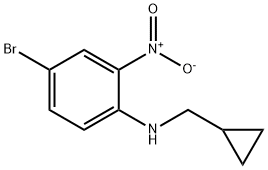
Benzenamine, 4-bromo-N-(cyclopropylmethyl)-2-nitro- synthesis
- Product Name:Benzenamine, 4-bromo-N-(cyclopropylmethyl)-2-nitro-
- CAS Number:886049-61-2
- Molecular formula:C10H11BrN2O2
- Molecular Weight:271.11
Yield:886049-61-2 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in ethanol at 96; for 15 h;
Steps:
F.1
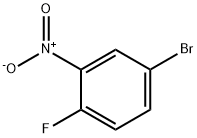
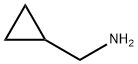
Step 1 Synthesis of 4-Bromo-N-(cyclopropylmethyl)-2-nitroaniline A mixture of 4-bromo-1-fluoro-2-nitrobenzene (9.25 g, 42 mmol), cyclopropanemethylamine (4.49 g, 63.1 mmol) and N,N-diisopropylethylamine (13.5 g, 105 mmol) in ethanol (100 mL) is stirred at 96° C. for 15 h. After cooling to room temperature, the mixture is concentrated under reduced pressure. The residue is purified by column chromatography (silica, eluent: ethyl acetate) to give 11.96 g of 4-bromo-N-(cyclopropylmethyl)-2-nitroaniline. Yield 100%, ES-MS: 271/273 [M+H].
References:
US2010/76029,2010,A1 Location in patent:Page/Page column 40