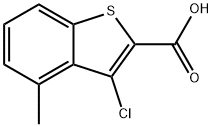
3-chloro-4-methylbenzo[b]thiophene-2-carboxylic acid synthesis
- Product Name:3-chloro-4-methylbenzo[b]thiophene-2-carboxylic acid
- CAS Number:923772-93-4
- Molecular formula:C10H7ClO2S
- Molecular Weight:226.68
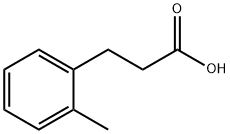
22084-89-5
111 suppliers
$5.00/250mg
![3-chloro-4-methylbenzo[b]thiophene-2-carboxylic acid](/CAS2/GIF/923772-93-4.gif)
923772-93-4
16 suppliers
$58.00/100mg
Yield:923772-93-4 65%
Reaction Conditions:
Stage #1: 3-(2-methylphenyl)propanoic acidwith pyridine;thionyl chloride at 150; for 5.5 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 20 - 60; for 0.5 h;
Steps:
1 Synthesis of 3-chloro-4-methylbenzo[b]thiophene-2-carboxylic acid
[Example 1] Synthesis of 3-chloro-4-methylbenzo[b]thiophene-2-carboxylic acid To a heated stirring mixture of 2-methylhydrocinnamic acid (20.00 g, 122 mmol), pyridine (1.0 mL, 12.2 mmol) and thionyl chloride (11.0 mL, 152 mmol) at 150°C there was slowly added dropwise thionyl chloride (32.0 mL, 396 mmol) at 150°C over a period of 2.5 hours, and stirring was continued for 3 hours. After 3 hours, the mixture was returned to room temperature, water (120 mL), 35% hydrochloric acid (12 mL) and tetrahydrofuran (200 mL) were added and the mixture was stirred at 60°C for 30 minutes. After 30 minutes, the tetrahydrofuran was distilled off under reduced pressure and the precipitated crystals were filtered out and dissolved in ethanol (100 mL) and water (300 mL) and stirred at 90°C for 1 hour, after which the solution was returned to room temperature and stirred overnight. The crystals that further precipitated were filtered out and recrystallized with a mixture of toluene (500 mL) and hexane (500 mL) to obtain 3-chloro-4-methylbenzo[b]thiophene-2-carboxylic acid (17.85 g, 78.7 mmol) (yield: 65%). 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.64(1H,d), 7.34(1H,t), 7.16(1H,d), 3.80(1H,br), 2.92(3H,s).
References:
EP1947096,2008,A1 Location in patent:Page/Page column 12-13