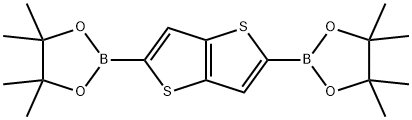
2,5-bis(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thieno[3,2-b]thiophene synthesis
- Product Name:2,5-bis(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thieno[3,2-b]thiophene
- CAS Number:924894-85-9
- Molecular formula:C18H26B2O4S2
- Molecular Weight:392.15
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
273 suppliers
$8.00/250mg
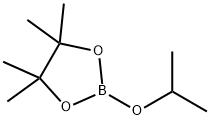
61676-62-8
311 suppliers
$10.00/5g
![2,5-bis(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thieno[3,2-b]thiophene](/CAS/GIF/924894-85-9.gif)
924894-85-9
50 suppliers
$53.00/100mg
Yield: 61.25%
Reaction Conditions:
Stage #1:thieno[3,2-b]thiophene with n-butyllithium in tetrahydrofuran;hexanes at -78; for 1.16667 h;Inert atmosphere;Cooling with ice;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexanes
Stage #3: with ammonium chloride in tetrahydrofuran;hexanes;water
Steps:
To a solution of thieno[3,2-b]thiophene (1 .5 g, 10.70 mmol) in THF (25.5 mL) at -78 °C under N 2 is added dropwise a solution of BuLi in hexanes (8.988 mL of 2.5 M, 22.47 mmol), stirred for 20 min, cooling bath is replaced with ice bath and stirred for 50 min. The resultant thick suspension is quenched with 2- isopropoxy-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane (4.181 g, 4.584 mL, 22.47 mmol). The reaction mixture is kept for overnight and then quenched with saturated aq. NH4Cl solution. After extraction with CH2Cl2 (2 x 100 mL), the combined extracts are washed with brine and dried (Na2S04). Organic solution is diluted with -20 mL of ethyl acetate, concentrated slowly on rotary evaporator until CH2Cl2 is removed. The resultant white fine crystals are collected by filtration. The solid is washed with heptanes and dried under high vacuum to afford 4,4,5,5-tetramethyl-2-[5-(4,4,5,5-tetramethyl-1 ,3,2- dioxaborolan-2-yl)thieno[3,2-b]thiophen-2-yl]-1 ,3,2-dioxaborolane (2.57 g, 6.554 mmol, 61.25%) as half-white solid. 1H NMR (400 MHz, CDCl3) δ 7.75 (s, 2H), 1.343 (s, 12H).
References:
VERTEX PHARMACEUTICALS INCORPORATED;DAS, Sanjoy Kumar;BENNANI, Youssef L. WO2011/119853, 2011, A1 Location in patent:Page/Page column 126-127
![2,5-DIBROMOTHIENO[3,2-B]THIOPHENE](/CAS/GIF/25121-87-3.gif)
25121-87-3
193 suppliers
$8.00/100mg

73183-34-3
559 suppliers
$6.00/5g
![2,5-bis(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thieno[3,2-b]thiophene](/CAS/GIF/924894-85-9.gif)
924894-85-9
50 suppliers
$53.00/100mg
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
273 suppliers
$8.00/250mg
25015-63-8
216 suppliers
$22.39/5G
![2,5-bis(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thieno[3,2-b]thiophene](/CAS/GIF/924894-85-9.gif)
924894-85-9
50 suppliers
$53.00/100mg
![2,5-DIBROMOTHIENO[3,2-B]THIOPHENE](/CAS/GIF/25121-87-3.gif)
25121-87-3
193 suppliers
$8.00/100mg

61676-62-8
311 suppliers
$10.00/5g
![2,5-bis(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thieno[3,2-b]thiophene](/CAS/GIF/924894-85-9.gif)
924894-85-9
50 suppliers
$53.00/100mg
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
273 suppliers
$8.00/250mg
![2,5-bis(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thieno[3,2-b]thiophene](/CAS/GIF/924894-85-9.gif)
924894-85-9
50 suppliers
$53.00/100mg