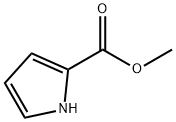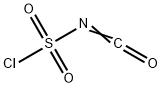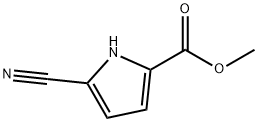
1H-Pyrrole-2-carboxylicacid,5-cyano-,methylester(9CI) synthesis
- Product Name:1H-Pyrrole-2-carboxylicacid,5-cyano-,methylester(9CI)
- CAS Number:937-19-9
- Molecular formula:C7H6N2O2
- Molecular Weight:150.13
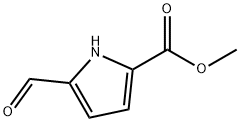
1197-13-3
88 suppliers
$8.00/100mg

937-19-9
29 suppliers
inquiry
Yield:937-19-9 71%
Reaction Conditions:
Stage #1: methyl 5-formyl-1H-pyrrole-2-carboxylatewith AMINOHIPPURATE SODIUM;hydroxylamine hydrochloride in methanol; for 1 h;Reflux;
Stage #2: with trichlorophosphate in N,N-dimethyl-formamide at -20 - 20; for 2.5 h;
Steps:
2 3.2
Methyl 5-cyano-1H-pyrrole-2-carboxylate (4)
NH2OH·HCl (0.58 g, 8.4 mmol) and NaOAc (0.63 g, 7.6 mmol) were added to a solution of methyl 5-formyl-1H-pyrrole-2-carboxylate
12
(1.14 g, 7.6 mmol) in anhydrous MeOH (8 mL), and the mixture was heated at the reflux temperature for 1 h.
The resulting solution was quenched with NaHCO3 aq, and the aqueous layer was extracted with CH2Cl2 for three times.
The combined organic layers were dried over anhydrous MgSO4 and concentrated in vacuo to give the corresponding aldoxime (1.11 g, <87%).
The crude oxime (5.8 g, 34 mmol) thus obtained was dissolved in anhydrous N,N-dimethylformamide (DMF, 28 mL), and the solution was added dropwise POCl3 (8.4 g, 55 mmol) at -20 °C.
After being stirred for 30 min at -20 °C and then at rt for 2 h, the reaction was quenched with water.
The aqueous layer was extracted with CH2Cl2 for three times.
The combined organic layers were washed with water for five times, dried over anhydrous MgSO4, and concentrated in vacuo to give the title compound (4.2 g, ~71%) as a colorless solid (mp=173.7-174.8 °C), Rf 0.23 (hexane/ethyl acetate=3:1).
1H NMR (400 MHz, CDCl3) δ 9.92 (br, 1H), 6.89 (dd, J=3.9, 2.5 Hz, 1H), 6.84 (dd, J=4.0, 2.6 Hz, 1H), 3.94 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.9, 126.7, 120.1, 115.2, 112.8, 105.7, 52.7; HRMS (EI) calcd for C7H6N2O2: M+, 150.0429. Found: m/z 150.0430.
References:
Yamada, Yuuya;Ebata, Shiro;Hiyama, Tamejiro;Nakao, Yoshiaki [Tetrahedron,2015,vol. 71,# 26-27,p. 4413 - 4417]

848499-68-3
0 suppliers
inquiry

937-19-9
29 suppliers
inquiry
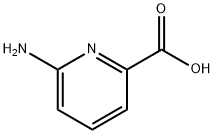
23628-31-1
247 suppliers
$6.00/1g

937-19-9
29 suppliers
inquiry
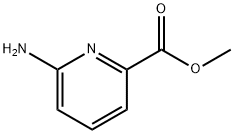
36052-26-3
185 suppliers
$6.00/1g

937-19-9
29 suppliers
inquiry