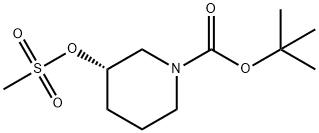
(S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE synthesis
- Product Name:(S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE
- CAS Number:940890-90-4
- Molecular formula:C11H21NO5S
- Molecular Weight:279.35

124-63-0
6 suppliers
$10.00/5g
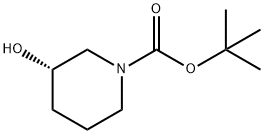
143900-44-1
541 suppliers
$5.00/1g

940890-90-4
40 suppliers
$68.00/250mg
Yield:940890-90-4 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 1 h;Inert atmosphere;
Steps:
1 [00224] tert-Butyl (3S)-3-methylsulfonyloxypiperidine-1-carboxylate
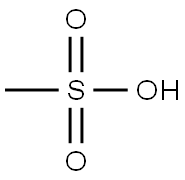
To a solution of (S)-1 -Boc-3-hydroxypiperidine (10.0 g, 49.7 mmol) and triethylamine (7.82 mL, 54.7 mmol in DCM (60 mL) under a nitrogen atmosphere, cooled at 0 °C, was added dropwise methanesulfonyl chloride (4.23 mL, 54.7 mmol). The mixture stirred for 1 h, diluted with water (60 mL) before being passed through a hydrophobic frit. The organic layer was concentrated under reduced pressure to afford fe/ -butyl (3S)-3-methylsulfonyloxypiperidine-1 -carboxylate (13.9 g, 49.7 mmol, 100% yield) as a colourless solid. H-NMR (400 MHz, DMSO-c/6): δ (ppm) 4.76-4.71 (m, 1 H, CH), 3.69-3.66 (m 1 H), 3.65-3.60 (m, 1 H), 3.49-3.43 (m, 1 H), 3.37-3.31 (m, 1 H), 3.07 (s, 3H, CH3), 2.02-1 .96 (m, 1 H), 1 .94-1 .91 (m, 1 H), 1 .87- 1 .80 (m, 1 H), 1 .59-1 .53, 1 .48-1 .45 (m, 9H)
References:
WO2014/188173,2014,A1 Location in patent:Paragraph 00224

124-63-0
6 suppliers
$10.00/5g
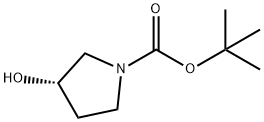
101469-92-5
339 suppliers
$6.00/5g

940890-90-4
40 suppliers
$68.00/250mg
75-75-2
601 suppliers
$10.00/5g

143900-44-1
541 suppliers
$5.00/1g

940890-90-4
40 suppliers
$68.00/250mg
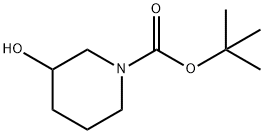
85275-45-2
366 suppliers
$7.00/5g

124-63-0
6 suppliers
$10.00/5g

940890-90-4
40 suppliers
$68.00/250mg
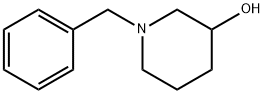
14813-01-5
322 suppliers
$9.00/1g

940890-90-4
40 suppliers
$68.00/250mg