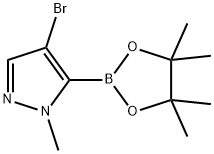
4-BroMo-1-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:4-BroMo-1-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:942070-88-4
- Molecular formula:C10H16BBrN2O2
- Molecular Weight:286.96
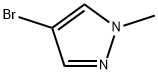
15803-02-8
323 suppliers
$5.00/1g
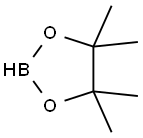
25015-63-8
215 suppliers
$22.39/5G

942070-88-4
41 suppliers
$30.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane;(1,5-cyclooctadiene)(methoxy)iridium(I) dimer in pentane at 20; for 0.166667 - 0.25 h;
Stage #2: with 4,4'-di-tert-butyl-2,2'-bipyridine in pentane for 0.333333 h;
Stage #3:4-bromo-1-methyl-1H-pyrazole at 20; for 5 h;Product distribution / selectivity;
Steps:
Preparation of 4-bromo-1-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole and 4-bromo-1-methyl-3,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole ; General Procedure A; In a dry glove box, an air-free flask, equipped with a magnetic stirring bar, is charged with [Ir(OMe)(COD)]2 (9.9 mg, 0.015 mmol, 3 mol % Ir), excess HBPin (typically, 1.5 equivs for monoborylation and 2.5), and pentane (typically 1.0 mL) and the mixture stirred at room temperature for 10 to 15 min. Then dtbpy (8.1 mg, 0.03 mmol, 3 mol %) is added to this mixture (with rinse with 0.5-1.0 mL) and reaction stirred for additional 20 min. The heteroaryl substrate (1 mmol, 1.0 equiv) is dissolved in pentane, THF or ether (typically 1-1.5 mL) and added to the active catalyst mixture. The reaction is stirred at room temperature until complete (monitored by TLC and GC-FID). Solvent is removed under reduced pressure, and the crude material is washed with pentane (3 mL portions until wash is colorless) to furnish the desired borylated product. Analytically pure sample is obtained by kuegelrohr distillation and used for spectroscopic and elemental analyses.; General procedure A was applied to 4-bromo-1-methylpyrazole (161.0 mg, 1.0 mmol, 1.0 equiv) and HBPin (218 μL, 192 mg, 1.5 mmol, 1.5 equiv) at room temperature for 5 h. The crude reaction mixture was washed with pentane (3.0 mL portions until the pentane wash is completely colorless) inside the glovebox. The washed product was dried to afford a 88:12 mixture of 4-bromo-1-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (m.p. 72-74° C.) and 4-bromo-1-methyl-3,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (m.p. 220-221° C.) (195 mg, 68%) as an off-white solid, mp 72-74° C. 1H, 13C NMR, gHMQC and gHMBC spectroscopy were used to assign the mono- and diborylated products as 4-bromo-1-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole and 4-bromo-1-methyl-3,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole respectively. 1H NMR (CDC13, 300 MHz): δ 7.43 (s, 1H), 4.03 (s, 3H, CH3), 1.33 (br s, 12H, CH3 of pinacolate); 11B NMR (CDC13, 96 MHz): δ 27.7. LRMS (% rel. int.): m/e 289(29), 288(99), 287(56), 286 M+ (100), 285(26), 208(11), 207(81), 206(21), 165(100), 164(31); Anal. Calcd for C10H16BBrN2O2: C, 41.85; H, 5.62; N, 9.76. Found: C, 41.66; H, 5.57; N, 9.71.
References:
Board of Trustees of Michigan State University US2008/91027, 2008, A1 Location in patent:Page/Page column 6-7
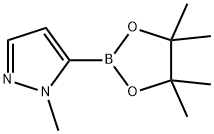
847818-74-0
262 suppliers
$6.00/1g

942070-88-4
41 suppliers
$30.00/250mg