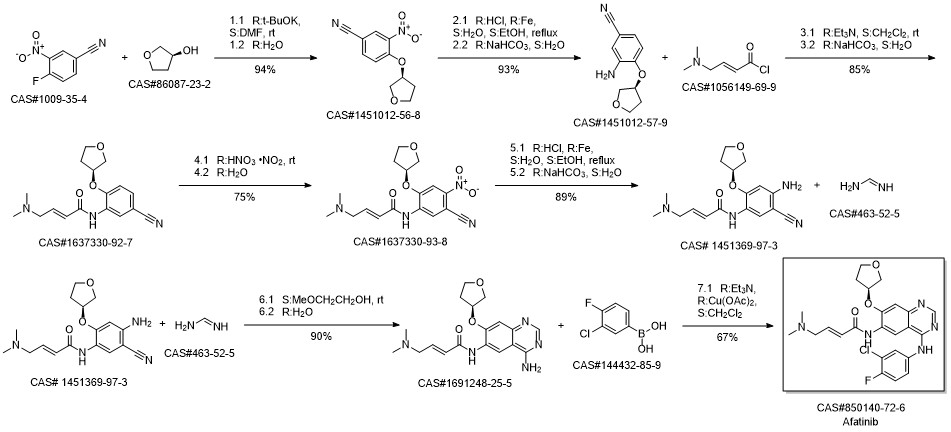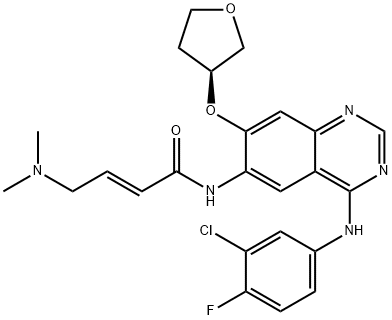
Afatinib synthesis
- Product Name:Afatinib
- CAS Number:850140-72-6
- Molecular formula:C24H25ClFN5O3
- Molecular Weight:485.94
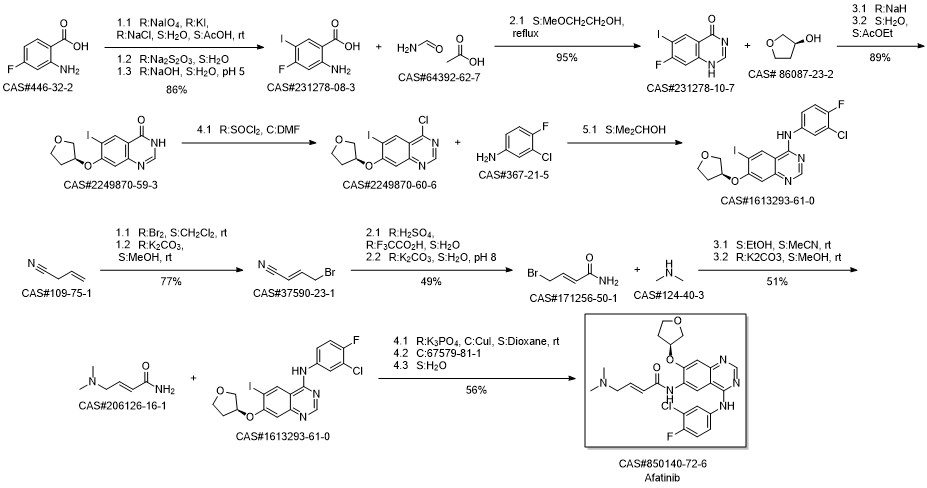
Kovacevic, Tatjana; Mesic, Milan; Avdagic, Amir; Zegarac, Miroslav. An alternative synthesis of the non-small cell lung carcinoma drug afatinib. Tetrahedron Letters. Volume 59. Issue 47. Pages 4180-4182. Journal; Online Computer File. (2018).
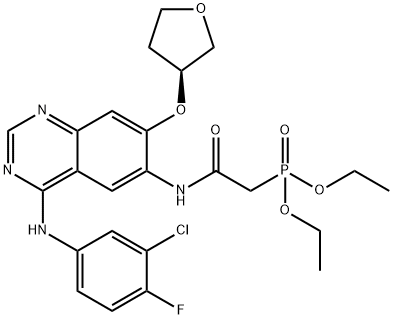
618061-76-0
84 suppliers
inquiry
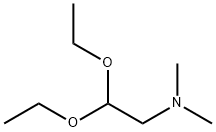
3616-56-6
210 suppliers
$5.00/5g

850140-72-6
327 suppliers
$35.00/5mg
Yield:850140-72-6 97.7%
Reaction Conditions:
Stage #1: dimethylaminoacetaldehyde diethyl acetalwith hydrogenchloride in water at 20 - 30;Inert atmosphere;
Stage #2: (S)-diethyl 2-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-ylamino)-2-oxoethylphosphonatewith lithium chloride;potassium hydroxide in tetrahydrofuran at -15 - 20; for 2.5 h;Product distribution / selectivity;Inert atmosphere;
Steps:
1
To a 50 ml single-neck round-bottom flask equipped with magnetic needle and nitrogen balloon was charged 5.0 ml (46.9 mmol) concentrated HCl, 5.0 ml water and the mixture was stirred at 30 °C. After 15 min, 5.3 ml (27.1 mmol) (dimethylamino)- acetaldehyde-diethyl acetal was added over a period of 5 min at 30 °C. The mixture was stirred at room temperature in an inert atmosphere overnight. The solution thus obtained was designated as reagent "A".A 250 ml two-neck round-bottom flask equipped with magnetic needle, thermometer and nitrogen balloon was charged with 6.0 g (10.85 mmol) diethyl (4-(3-chloro-4- fluorophenylam o)-7-(S)-(tetrahyc ^methyl)-phosphonate, 0.47 g (10.85 mmol), lithium chloride anhydrous and 25 ml THF. The mixture was cooled to -8 °C in an ice-salt bath and a cold solution of potassium hydroxide (4.7 g, 82.7 mmol dissolved in 24 ml water kept at -18°C) was added over the period of 15 min. Reagent "A" was added dropwise over the course of 30 min to the reaction mixture which was maintained at -7 °C and stirred at the same temperature for 1 h. The reaction was slowly allowed to come to 20 °C and stirred at this temperature for 45 min. 20 ml water were added and the mixture was extracted with 3 x 50 ml ethyl acetate. The combined extract was dried on sodium sulphate, evaporated and the resulting residue dried at 45 °C under vacuum to give a yellow solid. 200 ml water were added to the solid, the mixture was stirred for 1 h, filtered, washed with 200 ml water, dried on a rotary evaporator at 45 °C for 2 h to give 5.1 g (97.7%, 10.6 mmol) of an off- white solid. DSC shows two endothermic peaks at 95.9 °C and 138.6 °C.IR (cm"1): 3547.4, 2980.2, 2947.8, 2865.7, 2774, 1673.1, 1626.9, 1575.8, 1536.1,1500.1, 1455.7, 1430.5, 1397.0, 1233.4, 1147.1, 981.9, 852.1, 778.5 and 660.9.
References:
WO2012/121764,2012,A1 Location in patent:Page/Page column 49-50
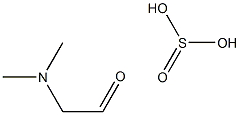
1413945-87-5
31 suppliers
$30.00/250mg

850140-72-6
327 suppliers
$35.00/5mg

618061-76-0
84 suppliers
inquiry

13804-54-1
0 suppliers
inquiry

850140-72-6
327 suppliers
$35.00/5mg