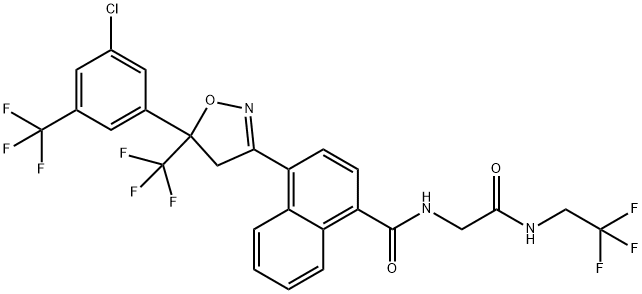
afoxolaner synthesis
- Product Name:afoxolaner
- CAS Number:1093861-60-9
- Molecular formula:C26H17ClF9N3O3
- Molecular Weight:625.87
![4-{3-[3-chloro-5-(trifluoromethyl)phenyl]-4,4,4-trifluorobut-2-enoyl}-N-{2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl}-1-naphthamide](/CAS/20200401/GIF/1125812-56-7.gif)
1125812-56-7
3 suppliers
inquiry

1093861-60-9
163 suppliers
$120.00/10mg
Yield:1093861-60-9 84%
Reaction Conditions:
with hydroxylamine sulfate;sodium hydroxide in tetrahydrofuran;water at 25;Product distribution / selectivity;
Steps:
7A.B
Step B: Preparation of 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-JV-[2-oxo-2-[(2,2,2- trifluoroethyl)amino]ethyl]- 1 -naphthalenecarboxamideA solution of sodium hydroxide (15.10 g of a 50% aqueous solution, 0.19 mmol) in water (total volume 67.5 mL) and a solution of hydroxylamine sulfate (7.75 g, 47.3 mmol) in water (total volume 67.5 mL) were added simultaneously to the product of Example 7A, Step A (51.90 g, 81.78 mmol) in tetrahydrofuran (300 mL) at 25 0C over 75 minutes. After the addition was complete, the mixture was stirred further for 180 minutes. The mixture was acidified to approximately pH 3 by addition of hydrochloric acid (concentrated, approximately 11 g). The aqueous layer was removed, and the remaining organic solution was heated to boiling. Acetonitrile was added, and the acetonitrile/tetrahydrofuran distillate was removed until the distillate temperature reached 82 0C, indicating that all of the tetrahydrofuran had been removed. The mixture was allowed to cool to 25 0C, and the acetonitrile was removed under reduced pressure. The residue was dissolved in acetonitrile (200 mL), cooled to 0 0C, and the resulting mixture was filtered to afford the title product as a white solid (43.45 g, 84% yield).The 1H NMR spectrum of the product was identical to the spectrum of the material prepared in Example 7, Step D.
References:
WO2009/126668,2009,A2 Location in patent:Page/Page column 54
![1-[3-Chloro-5-trifluoromethylphenyl]-2,2,2-trifluoroethanone](/CAS2/GIF/1125812-58-9.gif)
1125812-58-9
118 suppliers
$21.00/100mg

1093861-60-9
163 suppliers
$120.00/10mg
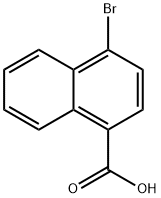
16650-55-8
152 suppliers
$12.00/1g

1093861-60-9
163 suppliers
$120.00/10mg
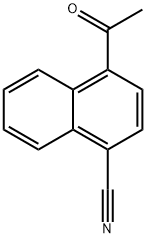
29139-00-2
10 suppliers
inquiry

1093861-60-9
163 suppliers
$120.00/10mg
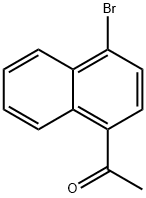
46258-62-2
92 suppliers
$24.00/100mg

1093861-60-9
163 suppliers
$120.00/10mg