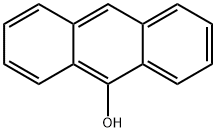
Anthracen-9-ol synthesis
- Product Name:Anthracen-9-ol
- CAS Number:529-86-2
- Molecular formula:C14H10O
- Molecular Weight:194.23
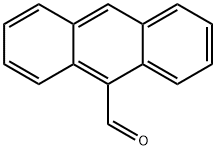
642-31-9

529-86-2
The general procedure for the synthesis of 9-hydroxyanthracene from 9-anthracenecarboxaldehyde is as follows: in a 1,000 mL round-bottomed flask equipped with a stirrer and thermometer, 20.00 g of 9-formylanthracene and 200 mL of acetic acid were added. After stirring the reaction mixture for 15 minutes, 45.68 g of sodium percarbonate was slowly added at room temperature. Subsequently, the reaction temperature was raised to 30 °C and the reaction mixture was continuously stirred, and the progress of the reaction was monitored by thin-layer chromatography (TLC; unfolding agent: n-hexane/ethyl acetate, 4:1). Upon completion of the reaction, the acetic acid was removed by distillation under reduced pressure and the reaction mixture was concentrated. To the concentrate, 200 mL of toluene was added and the toluene layer was washed sequentially with 100 mL of water, 100 mL of 10% sodium thiosulfate solution and 100 mL of 10% sodium hydroxide solution. Next, 22.75 g of methanol and 0.65 g of sulfuric acid were added to the toluene layer, and the resulting methyl formate and methanol were removed by distillation at atmospheric pressure. Then, 82.47 g of 10% sodium hydroxide solution was added to the toluene layer, and after stirring at room temperature, the toluene layer and the basic aqueous solution layer were separated using a partition funnel. The basic aqueous solution layer was washed several times with 20 mL of dichloromethane to remove small amounts of residual impurities (e.g., anthracene, 9-formylanthracene, etc.) until the aqueous layer was free of impurities by TLC. Subsequently, the basic aqueous solution layer was adjusted to a slightly acidic pH with concentrated sulfuric acid. the slightly reddish powder obtained was filtered and recrystallized in toluene to obtain pure 9-hydroxyanthracene (yield: 78%, purity: 99.56%).

642-31-9
341 suppliers
$10.00/5g

529-86-2
43 suppliers
$188.00/250mg
Yield: 78%
Reaction Conditions:
with acetic acid for 0.25 h;
Steps:
4 Example 4 Preparation of Hydroxy Compound (polycyclic aromatic is anthracene and R1 is hydrogen)
20.00 g of 9-formyl anthracene and 200 mL of acetic acid were added to a 1,000 mL round flask equipped with a stirrer and a thermometer. After the reaction was stirred for 15 minutes, 45.68 g of sodium percarbonate was added slowly at room temperature. Subsequently, the reaction temperature was raised to 30 DEG C, and the progress of the reaction progressed while stirring the reaction product was confirmed by TLC (thin layer chromatography; developing solution: n-hexane / ethyl acetate, 4/1). At the end of the reaction, acetic acid was distilled off under reduced pressure, and the reaction product was concentrated. 200 mL of toluene was added to the concentrated reaction product. 100 g of water, 100 g of 10% sodium thiosulfate and 100 g of 10% sodium hydroxide were sequentially added to the toluene layer to wash the toluene layer. 22.75 g of methanol and 0.65 g of sulfuric acid were added to the toluene layer at room temperature, and the resulting methylformate and methanol were removed by distillation under atmospheric pressure. Next, 82.47 g of 10% sodium hydroxide was added to the toluene layer, stirred at room temperature, and then the toluene layer and the aqueous alkaline solution layer were separated using a funnel. The alkaline aqueous solution layer was washed several times with 20 mL of dichloromethane to remove a small amount of impurities (anthracene, 9-formylanthracene, etc.) remaining in the water layer and analyzed with a water layer to wash with dichloromethane until no impurities were present. And repeated. Subsequently, the alkaline aqueous solution layer was adjusted to pH slightly acidic with concentrated sulfuric acid. The resulting reddish powder was filtered and then recrystallized in a toluene solvent to obtain pure 9-hydroxyanthracene (yield: 78%, purity: 99.56%).
References:
Im Gwang-min KR101661742, 2016, B1 Location in patent:Paragraph 0084; 0085
120-12-7
341 suppliers
$10.00/5g

529-86-2
43 suppliers
$188.00/250mg
84-65-1
585 suppliers
$5.00/100mg
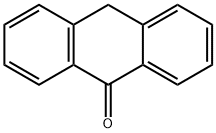
90-44-8
396 suppliers
$13.00/5g

529-86-2
43 suppliers
$188.00/250mg
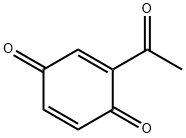
1125-55-9
2 suppliers
inquiry

529-86-2
43 suppliers
$188.00/250mg

120-12-7
341 suppliers
$10.00/5g
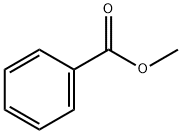
93-58-3
508 suppliers
$13.50/100 mL

529-86-2
43 suppliers
$188.00/250mg
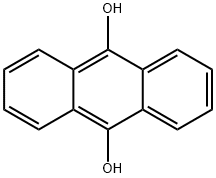
4981-66-2
37 suppliers
$87.00/250mg

90-44-8
396 suppliers
$13.00/5g
58343-58-1
2 suppliers
inquiry

84-65-1
585 suppliers
$5.00/100mg

100-52-7
974 suppliers
$17.30/2g