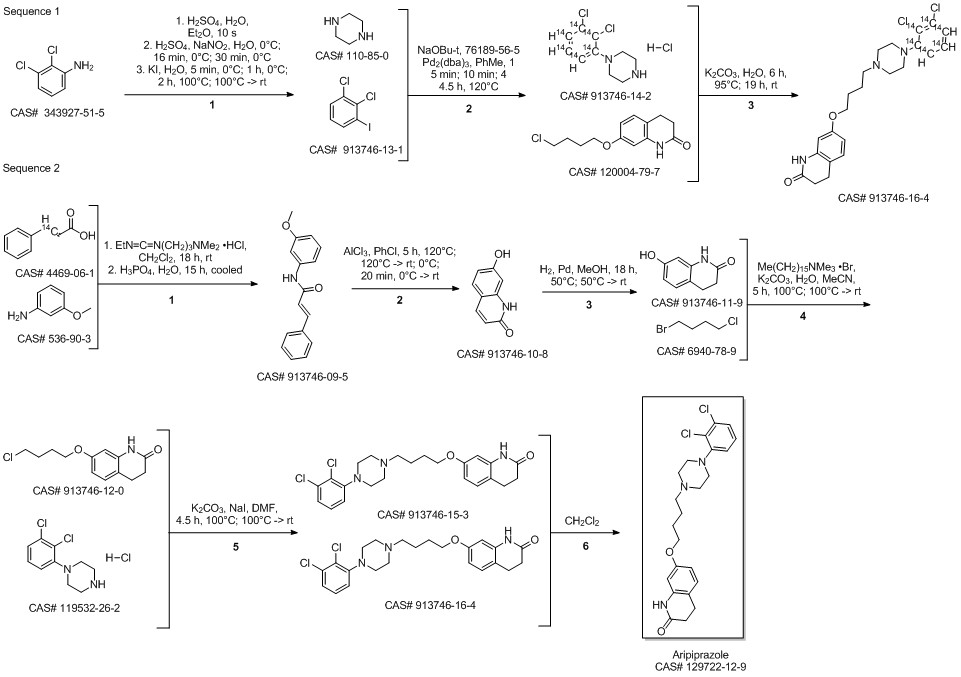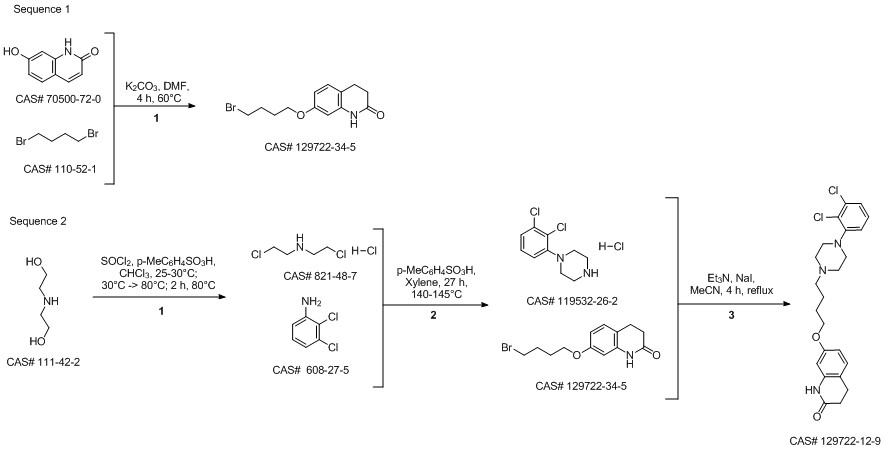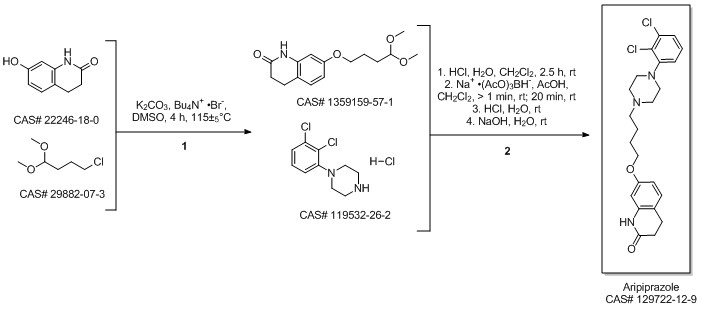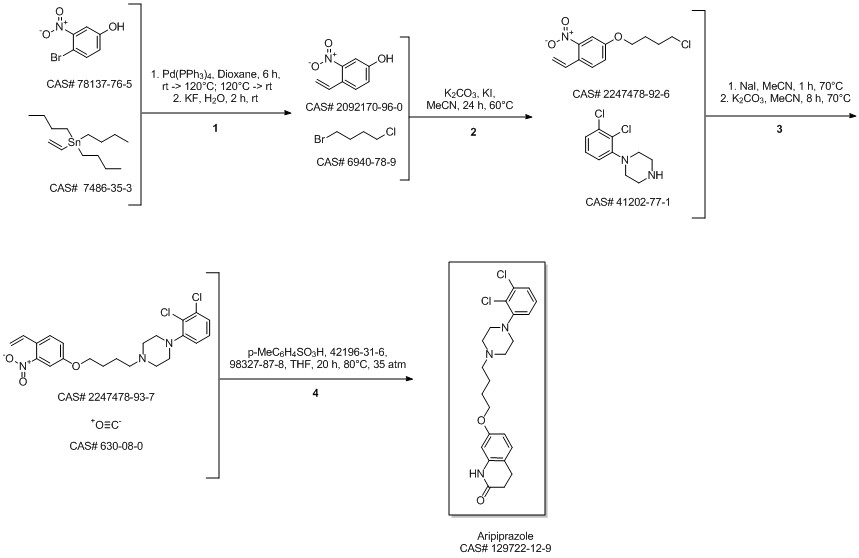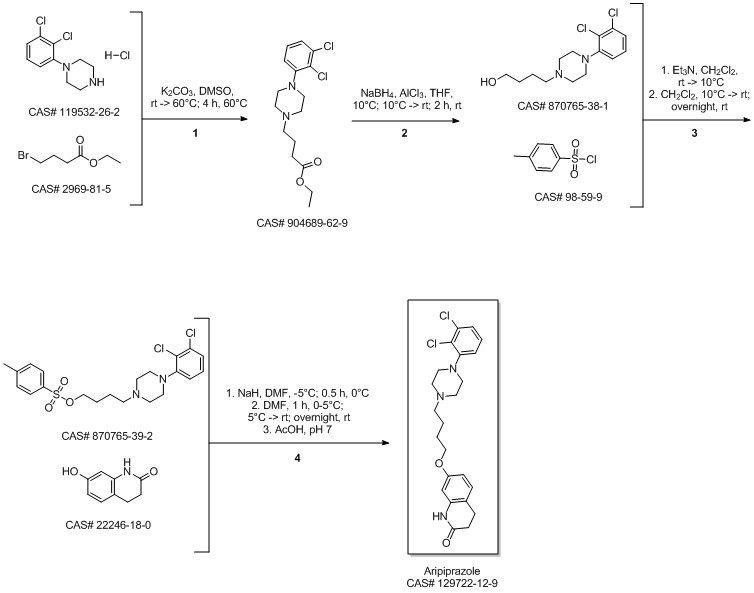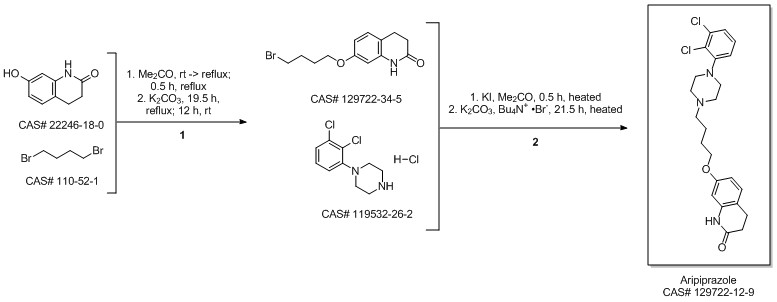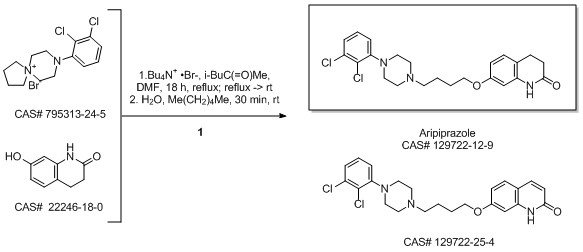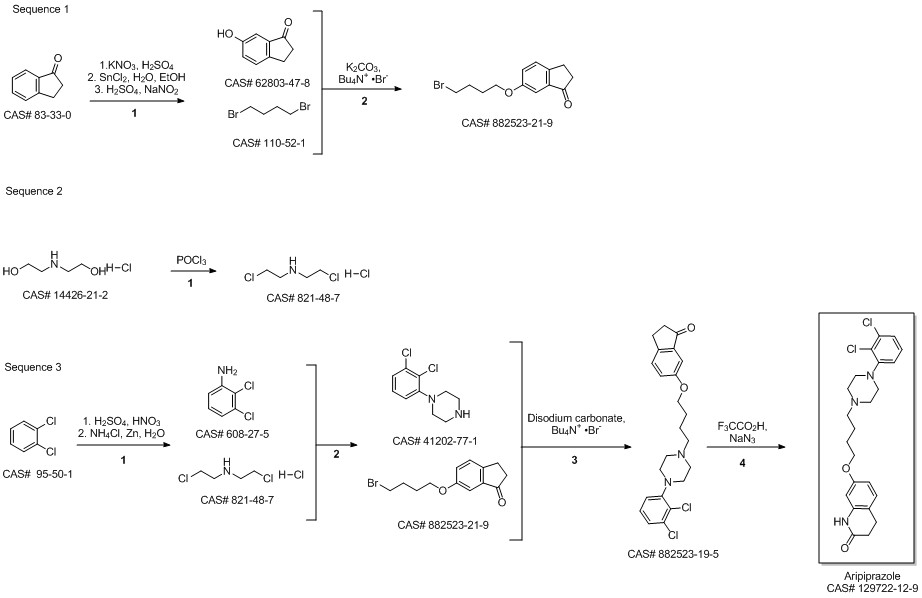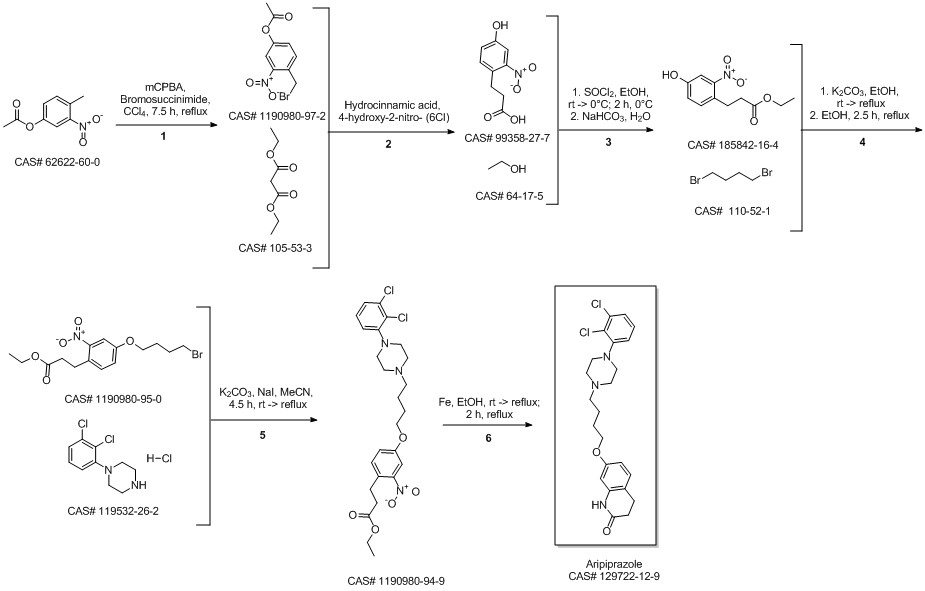
Aripiprazole synthesis
- Product Name:Aripiprazole
- CAS Number:129722-12-9
- Molecular formula:C23H27Cl2N3O2
- Molecular Weight:448.39
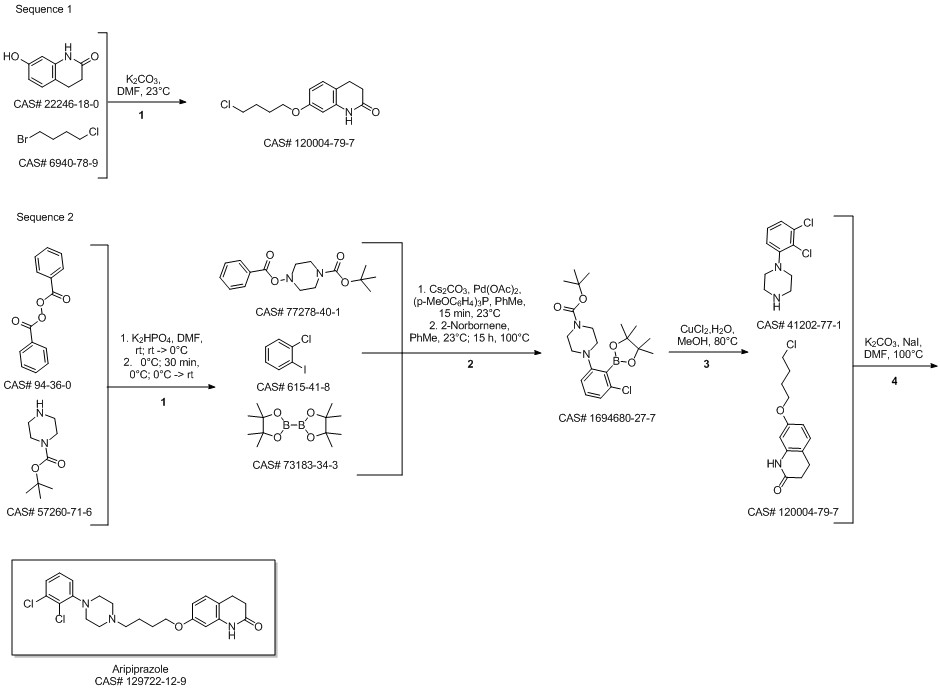
Shi, Hang; Babinski, David J.; Ritter, Tobias. Modular C-H Functionalization Cascade of Aryl Iodides. Journal of the American Chemical Society. Volume 137. Issue 11. Pages 3775-3778. 2015.
1026778-41-5
1 suppliers
inquiry

129722-12-9
556 suppliers
$5.00/100mg
Yield:129722-12-9 99.3%
Reaction Conditions:
at 100 - 120; for 21 h;Industrial scale;
Steps:
1 Example 1
Example 1
The aripiprazole hydrate A (powder) (44.29 kg) obtained in the Reference Example 4 was dried at 100° C. for 18 hours by using a hot air dryer and further heated at 120° C. for 3 hours, to obtain 42.46 kg (yield; 99.3%) of Anhydrous Aripiprazole Crystals B. These Anhydrous Aripiprazole Crystals B had a melting point (mp) of 139.7° C.
The Anhydrous Aripiprazole Crystals B obtained above had an 1H-NMR spectrum (DMSO-d6, TMS) which was substantially identical to the 1H-NMR spectrum shown in .
Specifically, they had characteristic peaks at 1.55-1.63 ppm (m, 2H), 1.68-1.78 ppm (m, 2H), 2.35-2.46 ppm (m, 4H), 2.48-2.56 ppm (m, 4H+DMSO), 2.78 ppm (t, J=7.4 Hz, 2H), 2.97 ppm (brt, J=4.6 Hz, 4H), 3.92 ppm (t, J=6.3 Hz, 2H), 6.43 ppm (d, J=2.4 Hz, 1H), 6.49 ppm (dd, J=8.4 Hz, J=2.4 Hz, 1H), 7.04 ppm (d, J=8.1 Hz, 1H), 7.11-7.17 ppm (m, 1H), 7.28-7.32 ppm (m, 2H) and 10.00 ppm (s, 1H).
The Anhydrous Aripiprazole Crystals B obtained above had a powder x-ray diffraction spectrum which was substantially the identical to the powder x-ray diffraction spectrum shown in .
Specifically, they had characteristic peaks at 2θ=11.0°, 16.6°, 19.3°, 20.3° and 22.1°.
The Anhydrous Aripiprazole Crystals B obtained above had remarkable infrared absorption bands at 2945, 2812, 1678, 1627, 1448, 1377, 1173, 960 and 779 cm-1 on the IR (KBr) spectrum.
References:
US9387182,2016,B2 Location in patent:Page/Page column 17
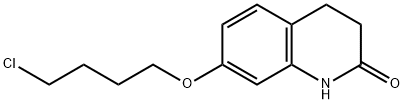
120004-79-7
159 suppliers
$60.00/2mg
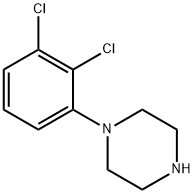
41202-77-1
254 suppliers
inquiry

129722-12-9
556 suppliers
$5.00/100mg
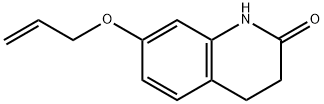
177419-01-1
1 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

41202-77-1
254 suppliers
inquiry

129722-12-9
556 suppliers
$5.00/100mg

41202-77-1
254 suppliers
inquiry
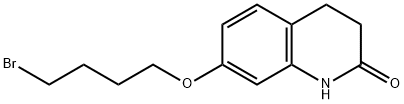
129722-34-5
348 suppliers
$5.00/250mg

129722-12-9
556 suppliers
$5.00/100mg
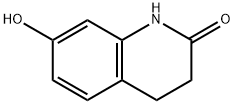
22246-18-0
612 suppliers
$5.00/5g

41202-77-1
254 suppliers
inquiry

110-56-5
293 suppliers
$15.00/25mL

129722-12-9
556 suppliers
$5.00/100mg