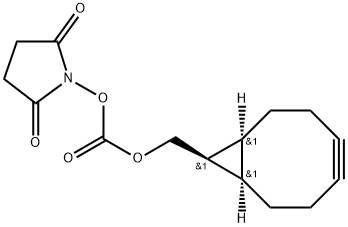
BCN-Osu synthesis
- Product Name:BCN-Osu
- CAS Number:1426827-79-3
- Molecular formula:C15H17NO5
- Molecular Weight:291.3
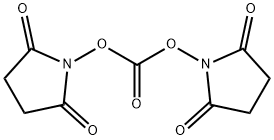
74124-79-1
451 suppliers
$5.00/5g
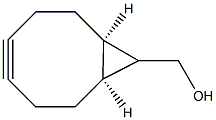
1263166-90-0
73 suppliers
$54.00/50mg

1426827-79-3
61 suppliers
$156.00/10MG
Yield:1426827-79-3 60%
Reaction Conditions:
with triethylamine in acetonitrile at 20;Inert atmosphere;
Steps:
1.S22 Synthesis of [(1R,8S,9S)-bicyclo[6.1.0]non-4-yn-9-yl]methyl 2,5-dioxopyrrolidin-1-yl carbonate (BCN-OSu)
(lR,8^,95)-bicyclo[6.1.0]non-4-yn-9-ylmethanol (1.48 g, 9.9 mmol) and Ν,Ν'- Disuccinimidyl carbonate (5.1 g, 19.9 mmol, 2x) were dissolved in acetonitrile (75 mL) under a nitrogen atmosphere. Triethylamine (3.3g, 4.5 mL, 32 mmol) was added and the reaction was stirred overnight at room temperature. The reaction was diluted with 1 : 1 ethyl acetate: ether (200 mL) and washed with water (6 x 100 mL) and brine (2 x 50 mL). The organic layer was dried over MgS04, filtered, and concentrated in vacuo. The product was purified on a silica flash column (3 : 1 hexanes:ethyl acetate) to yield the product [(lR,8S,9S)-bicyclo[6.1.0]non-4-yn-9-yl]methyl 2,5-dioxopyrrolidin-l-yl carbonate (denoted BCN-OSu) as a white solid (1.72 g, 5.9 mmol, 60% yield). 1H NMR (500 MHz, CDC13): δ 4.48 (d, J=8.4 Hz, 2H), 2.87 (s, 4H), 2.30 (m, 6H), 1.57 (m, 3H), 1.09 (m, 2H). These spectral data matched those previously reported.
References:
WO2018/57941,2018,A1 Location in patent:Page/Page column 39; 82; 83
111-78-4
151 suppliers
$10.00/25g

1426827-79-3
61 suppliers
$156.00/10MG

111-78-4
151 suppliers
$10.00/25g

1426827-79-3
61 suppliers
$156.00/10MG
![Ethyl (1α,8α,9β)-bicyclo[6.1.0]non-4-ene-9-carboxylate](/CAS/20211123/GIF/1263291-39-9.gif)
1263291-39-9
25 suppliers
$280.00/250mg

1426827-79-3
61 suppliers
$156.00/10MG