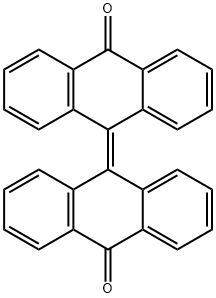
Bianthrone synthesis
- Product Name:Bianthrone
- CAS Number:434-85-5
- Molecular formula:C28H16O2
- Molecular Weight:384.43
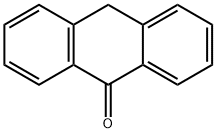
90-44-8
371 suppliers
$13.00/5g

434-85-5
94 suppliers
$10.00/100mg
Yield: 45%
Reaction Conditions:
with C12A7 electride in water;acetonitrile at 100; for 12 h;
Steps:
19; 2
Into a mixed solvent of water and cyanomethane (1:4) were put 10 mg of anthrone, and 164 mg of a C12A7 electride, and then the solution was put into an eggplant type flask 10 mL in volume. In the atmosphere of nitrogen gas, the active components were caused to react at 100°C for 12 hours while the solution was stirred. Next, the reaction solution was transferred to an eggplant type flask 50 mL in volume, and hydrochloric acid (1 N, 7 mL) was added thereto. Thereafter, thereto was added ethyl acetate (20 mL) and then the product was extracted. This extracting process was repeated 3 times, and then the product was washed with sodium bicarbonate solution and sodium chloride solution. Thereto was added magnesium sulfate to adsorb water, thereby removing water. Next, magnesium sulfate was filtrated off, and the solvent was distilled off. The resultant was purified by column chromatography (silica gel) to yield a compound having a purity of more than 98%. The identification of the compound was attained through the H1 nuclear magnetic resonance spectrum thereof. The compound was dianthrone. The yield of the product was 45%, which was calculated from the weight of the product.
References:
Japan Science and Technology Agency EP2067761, 2009, A1 Location in patent:Page/Page column 4-5; 13; 15-16
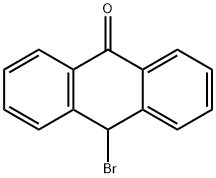
1560-32-3
4 suppliers
inquiry

434-85-5
94 suppliers
$10.00/100mg
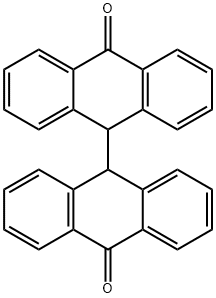
434-84-4
37 suppliers
$57.00/1g

434-85-5
94 suppliers
$10.00/100mg
![9,9'-Bi[anthracen-10-ol]](/CAS/GIF/16014-05-4.gif)
16014-05-4
0 suppliers
inquiry

434-85-5
94 suppliers
$10.00/100mg

90-44-8
371 suppliers
$13.00/5g
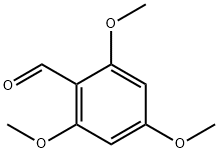
830-79-5
263 suppliers
$7.00/1g

434-85-5
94 suppliers
$10.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry