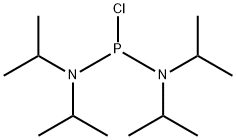
BIS(DIISOPROPYLAMINO)CHLOROPHOSPHINE synthesis
- Product Name:BIS(DIISOPROPYLAMINO)CHLOROPHOSPHINE
- CAS Number:56183-63-2
- Molecular formula:C12H28ClN2P
- Molecular Weight:266.79
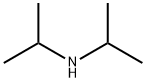
108-18-9
351 suppliers
$10.00/1g

56183-63-2
118 suppliers
$16.80/250MG
Yield:56183-63-2 74%
Reaction Conditions:
Stage #1: diisopropylamine in acetonitrile at 0; for 0.5 h;
Stage #2: with phosphorus trichloride in acetonitrile at 0;
Steps:
A 5 L three neck round bottom flask was equipped with a Friedrich's condenser, a ground glass stirrer bearing, a silicon rubber septum, and placed under dry argon. Two liters of anhydrous diisopropylamine (1.6 kg, 15.9 mol) were added to the flask and diluted by addition of two liters anhydrous acetonitrile. The solution was mixed with a mechanical stirrer attached to a glass rod and a Teflon blade. An ice-water bath was placed under the flask and the solution allowed to cool with stirring for 30 min. Phosphorus trichloride (313 g, 2.3 mol) was placed in a dry two liter flask and one liter of anhydrous acetonitrile added. This phosphorus trichloride solution was then added slowly by cannulation to the vigorously stirred solution of diisopropylamine. Once addition was complete, the ice bath was removed and the reaction mixture stirred overnight. Complete conversion of the phosphorus trichloride (δ 201 ppm) to product (δ 134 ppm) was monitored by 31P NMR. The reaction mixture was filtered to remove diisopropylamine hydrochloride and the precipitate washed with anhydrous ether. The combined filtrates were concentrated in vacuo to a semi-crystalline solid. Anhydrous hexanes (1.5 L) were added to the flask and the mixture heated. The hot liquid was filtered through a Schlenk filter funnel to remove residual amine hydrochloride and the resulting clear liquid concentrated in vacuo to half the original volume. The product was then isolated by crystallization, filtration, and drying in vacuo to yield 447 g (74% yield). 31P NMR (CD3CN) δ 134.4 (s); Electron Impact Mass Spectrometry gave a molecular ion of 267 m/e.
References:
US2007/99859,2007,A1 Location in patent:Page/Page column 17
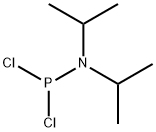
921-26-6
82 suppliers
$80.00/1g

108-18-9
351 suppliers
$10.00/1g

56183-63-2
118 suppliers
$16.80/250MG