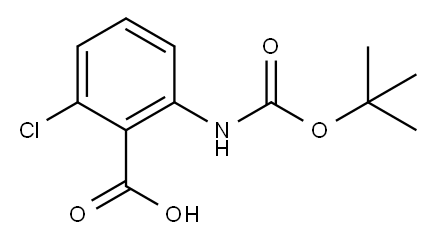
BOC-2-AMINO-6-CHLOROBENZOIC ACID synthesis
- Product Name:BOC-2-AMINO-6-CHLOROBENZOIC ACID
- CAS Number:616224-61-4
- Molecular formula:C12H14ClNO4
- Molecular Weight:271.7

24424-99-5
815 suppliers
$13.50/25G

616224-61-4
15 suppliers
$212.00/1g
Yield:616224-61-4 90%
Reaction Conditions:
Stage #1: 2-chloro-6-aminobenzoic acidwith sodium hexamethyldisilazane in tetrahydrofuran; for 0.25 h;
Stage #2: di-tert-butyl dicarbonate in tetrahydrofuran; for 3 h;
Steps:
2.16 Reference Example 2-16; Synthesis of 2-(Boc)amino-6-chlorobenzoic acid
After dissolving 2-amino-6-chlorobenzoic acid (1.13 g, 6.59 mmol) in tetrahydrofuran (5.0 mL), a solution of sodium bistrimethylsilylamide/1.0 M in THF (19.8 mL, 19.8 mmol) was added dropwise. After stirring this mixture for 15 minutes, a solution of (Boc)2O (1.82 mL, 7.91 mmol) in tetrahydrofuran (2.0 mL) was added dropwise, and the mixture was stirred for 3 hours. Water (20 mL) and 1N hydrochloric acid (about 25 mL) were added to the reaction mixture to adjust the pH to approximately 4. It was then extracted with ethyl acetate (40 mL x 3 times), and the obtained organic layer was washed with water (50 mL x 2 times) and saturated brine (50 mL), and then dried over anhydrous magnesium sulfate. The dried product was concentrated under reduced pressure to obtain a concentrated residue, which was then purified by silica gel column chromatography (methylene chloride:methanol:acetic acid = 95:5:1) to obtain 2-(Boc)amino-6-chlorobenzoic acid. The compound was identified by LC-MS and NMR. Yield: 1.62 g (90%), M+23 = 294.0.1H-NMR (270 MHz, CDCl3): δ8.40(1H,s), 8.04(1H,d,J=8.2Hz), 7.35(1H,t,J=8.2 Hz), 7.13(1H,d,J=8.2 Hz), 1.52(9H,s) ppm
References:
EP1502916,2005,A1 Location in patent:Page 456
129822-39-5
13 suppliers
inquiry

616224-61-4
15 suppliers
$212.00/1g

24424-99-5
815 suppliers
$13.50/25G
2148-56-3
360 suppliers
$5.00/10g

616224-61-4
15 suppliers
$212.00/1g

2148-56-3
360 suppliers
$5.00/10g

269391-47-1
8 suppliers
$324.00/1g

616224-61-4
15 suppliers
$212.00/1g

24424-99-5
815 suppliers
$13.50/25G
87-60-5
407 suppliers
$8.00/25g

616224-61-4
15 suppliers
$212.00/1g