Brinzolamide synthesis
- Product Name:Brinzolamide
- CAS Number:138890-62-7
- Molecular formula:C12H21N3O5S3
- Molecular Weight:383.51
Graphical Synthetic Routes of Olanzapine
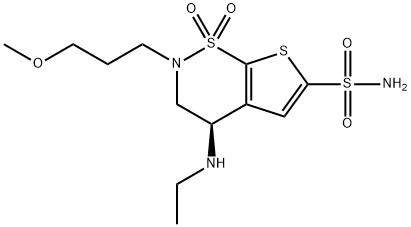
150937-43-2
20 suppliers
$139.90/2 mg

138890-62-7
419 suppliers
$5.00/10mg
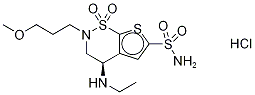
Yield:138890-62-7 500 g
Reaction Conditions:
with triethylamine in water; pH=7 - 8.5 at 25 - 30; for 2 h;
Steps:
1; 1 Example 1: Preparation of Brinzolamide free base from hydrochloride salt:
Example 1: Preparation of Brinzolamide free base from hydrochloride salt: Brinzolamide Hydrochloride (750 g) was dissolved in 5 volume water and filtered through celite bed to remove insoluble particles. Further, the solution was adjusted the pH to 7-8.5 with Triethylamine under stirring. Stirring was continued for additional 2 hours at 25 to 30°C followed by chilling the reaction mass at 0 to 5°C and stirred for 1 hour. The solids were filtered and washed with 1 volume of water. Wet cake was dried at 40°C under vacuum to yield Brinzolamide free base (500 g, HPLC purity: 98.5%).
References:
BIOCON LIMITED;KOTHAKONDA, Kiran Kumar;VENKATA, Srinivas Pullela;RAJMAHENDRA, Shanmughasamy;MHETRE, Sandeep Madhukar;CHANDAVARKAR, Arun WO2013/114397, 2013, A2 Location in patent:Page/Page column 7; 9
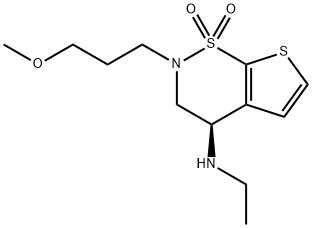
![(S)-3,4-Dihydro-4-hydroxy-2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide 1,1-dioxide](/CAS/20180808/GIF/154127-42-1.gif)
154127-42-1
189 suppliers
$6.00/100mg
75-04-7
370 suppliers
$10.00/20mg

138890-62-7
419 suppliers
$5.00/10mg
1029324-92-2
1 suppliers
inquiry

138890-62-7
419 suppliers
$5.00/10mg
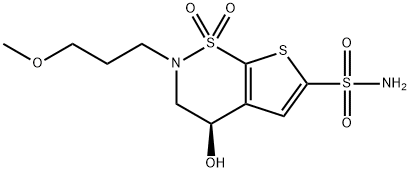
165117-54-4
33 suppliers
inquiry

75-04-7
370 suppliers
$10.00/20mg

138890-62-7
419 suppliers
$5.00/10mg
![(S)-3,4-Dihydro-4-hydroxy-2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide 1,1-dioxide](/CAS/20180808/GIF/154127-42-1.gif)
154127-42-1
189 suppliers
$6.00/100mg

138890-62-7
419 suppliers
$5.00/10mg