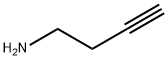
BUT-3-YN-1-AMINE synthesis
- Product Name:BUT-3-YN-1-AMINE
- CAS Number:14044-63-4
- Molecular formula:C4H7N
- Molecular Weight:69.11
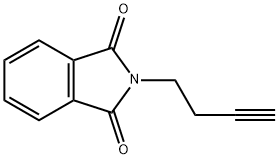
14396-90-8
64 suppliers
$57.00/2 g

14044-63-4
80 suppliers
$40.00/250mg
Yield:-
Reaction Conditions:
with hydrazine in methanol at 20;
Steps:
1 SS-1-97B:
To a stirred solution of SS-1-95 (180 mg, 0.9 mmol) in MeOH (5 mL) was added N2H4 (0.06 mL, 1.13 mmol). The resulting mixture was stirred at room temperature for 16 h. Then participate was filtered off, and the filtrate was quenched with water (5 mL), and acidified to pH 2 with 2 N HC1. The solution was concentrated under vacuum to afford SS-1-97A as white powder. The crude product was used directly into the next step. To a stirred solution of SS-1-97A in DCM (5 mL) was added TEA (0.37 mL, 2.7 mmol) and benzoyl chloride (252 mg, 1.8 mmol) at 0 °C. Then the resulting mixture was stirred at the same temperature for 30 mm. The reaction was quenched with water (5 mL), and extracted with DCM (3 x 10 mL). The combined organic extracts were washed with brine (40 mL), dried over sodium sulfate, and concentrated under vacuum. The crude product was purified by flash chromatography (0 - 50% EtOAc/Hexene), and the title compound was obtained as white powder (140 mg, 90%). ‘H NMR (400 IVIHz, Acetone-cl6) 7.93 (dd, J= 5.3, 3.2 Hz, 3H), 7.58 -7.53 (m, 1H), 7.49 (dd, J 8.1, 6.6 Hz, 2H), 3.57 (td, J= 7.1, 6.0 Hz, 2H), 2.55 (td, J 7.1, 2.7 Hz, 2H), 2.43 (t, J 2.7 Hz, 1H).
References:
THE GEORGE WASHINGTON UNIVERSITY;THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS;VILLAGRA, Alejandro;KOZIKOWSKI, Alan P.;SHEN, Sida WO2018/183701, 2018, A1 Location in patent:Paragraph 0244; 0248; 0252; 0256
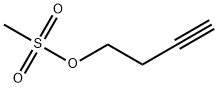
72486-09-0
31 suppliers
inquiry

14044-63-4
80 suppliers
$40.00/250mg

927-74-2
405 suppliers
$6.00/5g

14044-63-4
80 suppliers
$40.00/250mg
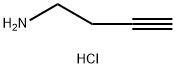
88211-50-1
129 suppliers
$21.00/250mg

14044-63-4
80 suppliers
$40.00/250mg
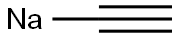
1066-26-8
90 suppliers
$48.00/50g
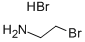
2576-47-8
464 suppliers
$8.00/25g

14044-63-4
80 suppliers
$40.00/250mg