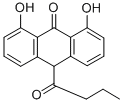
Butantrone synthesis
- Product Name:Butantrone
- CAS Number:75464-11-8
- Molecular formula:C18H16O4
- Molecular Weight:0
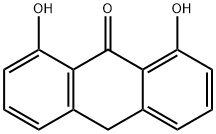
1143-38-0
295 suppliers
$15.00/100mg
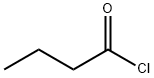
141-75-3
333 suppliers
$12.32/25G

75464-11-8
6 suppliers
inquiry
Yield:-
Reaction Conditions:
with pyridine in water;toluene;
Steps:
13.a (a)
(a) 1,8-Dihydroxy-10-butyrylanthrone To a stirred suspension of 51 g (0.22 mol) of 1,8-dihydroxyanthrone in 1.5 liters of toluene, kept under an inert atmosphere and shielded from light, 23.8 cm3 of pyridine was first added all at once and then 27.4 cm3 of butyryl chloride was added dropwise over 15 minutes. The mixture was then heated to a temperature of 80°-90° C. for 1 hour. Subsequently, the same quantities as before of pyridine and butyryl chloride (i.e., a total of 2.7 molar equivalents for pyridine and of 2.4 molar equivalents for the acid chloride) were added at room temperature. The reaction mixture was then reheated to 80°-90° C. for 1 hour. After being cooled to room temperature, the mixture was poured into 500 cm3 of acidulated water. The organic phase was decanted, washed with water until the washings became neutral and dried over magnesium sulfate. This phase was then fractionated by chromatography on a silica gel column, using toluene as the mobile phase. The eluted phases were concentrated and poured into hexane, thus causing crystallization of 1,8-dihydroxy-10-butyrylanthrone. The solvent was sucked from this product, which was then dried to yield 20 g of yellow crystals having a melting point of 138° C. Analysis: C18 H16 O4 Calc.: C 72.96, H 5.44, O 21.60; Found: C 72.75, H 5.48, O 21.38.
References:
US4696941,1987,A