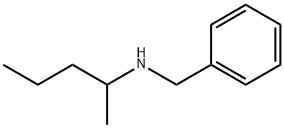
CHEMBRDG-BB 4024859 synthesis
- Product Name:CHEMBRDG-BB 4024859
- CAS Number:61806-76-6
- Molecular formula:C12H19N
- Molecular Weight:177.29
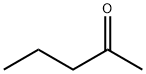
107-87-9
328 suppliers
$14.00/25mL
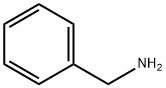
100-46-9
461 suppliers
$5.00/5G

61806-76-6
13 suppliers
$45.00/100mg
Yield:61806-76-6 99%
Reaction Conditions:
Stage #1: 2-Pentanone;benzylaminewith formic acid;chlorido(8-quinolinolato-k2N,O)(η5-pentamethylcyclo-pentadienyl)rhodium(III) in ethyl acetate at 0 - 40;Inert atmosphere;Cooling with ice;Schlenk tube;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;Product distribution / selectivity;
Steps:
14
Example 14; Synthesis of N-benzyl-2-penthylamine by reductive amination reaction of 2-pentanone and benzylamine; Under an argon-gas atmosphere, 532 μL (5.0 mmol) of 2-pentanone (MW: 86.13), 572 μL (5.25 mmol) of benzylamine (MW: 107.15), and 0.5 mL of dehydrated ethyl acetate (Kanto Chemical Co., Inc.) were added to a 20-mL Schlenk tube and ice-cooled. 566 μL (15.0 mmol) of formic acid (MW: 46.03) was added and stirred for approximately 5 min, then the ice bath was removed, and as reaction examples 1-6, 0.0025 mmol of each of the complexes shown in FIG. 1 was added and stirred at 40° C. for 18 hr. After the reaction was completed, a saturated sodium hydrogen carbonate solution was added and stirred for approximately 5 min, then the product was extracted with ether. The organic phase was analyzed by GC to calculate conversion rates and the reaction yields. Results of the analysis were summarized in Table 1. Compared to Comparative examples 1 and 2, reactivities significantly increased when the inventive complexes of the same metals were used, suggesting the efficacy of the invention.
References:
US2010/234596,2010,A1 Location in patent:Page/Page column 12

625-30-9
18 suppliers
inquiry
100-51-6
1370 suppliers
$5.00/100g

61806-76-6
13 suppliers
$45.00/100mg

107-87-9
328 suppliers
$14.00/25mL
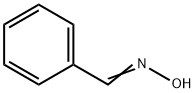
932-90-1
128 suppliers
$17.67/1gm:

61806-76-6
13 suppliers
$45.00/100mg
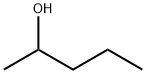
6032-29-7
167 suppliers
$14.00/5mL
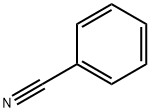
100-47-0
357 suppliers
$14.14/25gm:

61806-76-6
13 suppliers
$45.00/100mg

107-87-9
328 suppliers
$14.00/25mL

61806-76-6
13 suppliers
$45.00/100mg