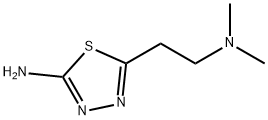
CHEMBRDG-BB 6038299 synthesis
- Product Name:CHEMBRDG-BB 6038299
- CAS Number:14068-78-1
- Molecular formula:C6H12N4S
- Molecular Weight:172.25
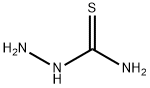
79-19-6
487 suppliers
$14.00/1g
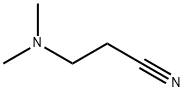
1738-25-6
118 suppliers
$29.00/25mL

14068-78-1
8 suppliers
$45.00/50mg
Yield:14068-78-1 33%
Reaction Conditions:
Stage #1: thiosemicarbazide;3-dimethylaminopropiononitrilewith trifluoroacetic acid at 80; for 6 h;
Stage #2: with potassium carbonate in water;
Steps:
9.i
Step (i) of Reference Example 9: 5-(2-(Dimethylamino)ethyl)-1,3,4-thiadiazol-2-amine A solution of 2.00 ml (17.7 mmol) of 3-(dimethylamino)propanenitrile and 1.70 g (18.6 mmol) of thiosemicarbazide in trifluoroacetic acid (5 ml) was heated at 80°C for 6 hr. The reaction solution was cooled to room temperature and was then diluted with water. The diluted solution was rendered weakly basic by the addition of a 10% aqueous potassium carbonate solution. The solution was extracted with chloroform, and the extract was dried over anhydrous sodium sulfate. The filtrate was concentrated, and the residue was washed with diethyl ether-ethyl acetate to give 1.00 g (yield 33%) of the title compound. 1H-NMR (400 MHz, CDCl3) δ: 2.29 (6H, s), 2.61 (2H, t, J = 6.8 Hz), 3.06 (2H, t, J = 6.8 Hz), 5.21 (2H, br). MS (FAB) m/z 173 [M+H]+.
References:
EP2151447,2010,A1 Location in patent:Page/Page column 44