Cholic acid synthesis
- Product Name:Cholic acid
- CAS Number:81-25-4
- Molecular formula:C24H40O5
- Molecular Weight:408.58
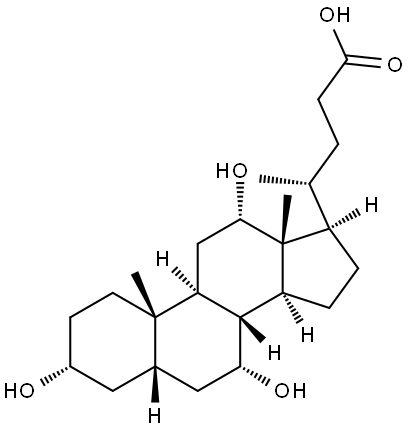
911-40-0
64 suppliers
$39.39/50MG

81-25-4
504 suppliers
$5.00/100mg
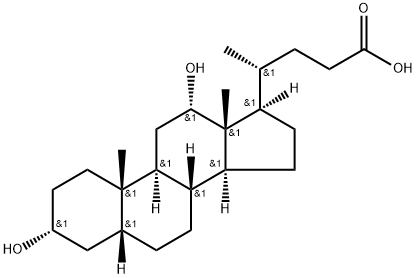
![4-[(5S,7S,8S,10S,13R,17R)-3,7,12-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid](/CAS/GIF/2955-27-3.gif)
2955-27-3
87 suppliers
$80.00/1mg
Yield:2955-27-3 80% ,81-25-4 10%
Reaction Conditions:
with sodium in propan-1-ol; for 3 h;Reflux;
Steps:
2.2.2. 3α,7β,12α-Trihydroxy-5β-cholane-24-oic acid 4
To a solution of 2.50 g (6.1 mmol) of 3α,12α-dihydroxy-7-keto-5β-cholane-24-oic acid 2 in 20 ml of anhydrous n-propanol was added 5.09 g (220.0 mmol) of sodium metal. Reaction mixture was refluxed and progress of reaction was monitored by TLC. Reaction was completed in 3 h. The reaction mixture was cooled to room temperature and gradually diluted with 20 ml of water, acidified with dilute hydrochloric acid, and extracted with (2 × 30 ml) of ethyl acetate. Organic layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure to get residue which was further purified by column chromatography on silica gel using methanol-chloroform (5:95). Yield: 2 g (80%); Mp: 144 °C [lit.12 145 °C]; IR (KBr): 1704 (C0), 3448 (OH), 1042, 1010 cm-1;1H NMR (300 MHz, CDCl3): δ 0.69 (s, 3H, H-18), 0.91 (s, 3H, H-19), 3.51 (br m, 2H, H-3β and H-7α), 3.91(m, 1H, H-12β).
References:
Dangate, Prasad S.;Salunke, Chetan L.;Akamanchi, Krishnacharya G. [Steroids,2011,vol. 76,# 12,p. 1397 - 1399] Location in patent:experimental part
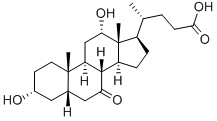
81-23-2
242 suppliers
$28.00/1g

81-25-4
504 suppliers
$5.00/100mg

911-40-0
64 suppliers
$39.39/50MG

81-25-4
504 suppliers
$5.00/100mg
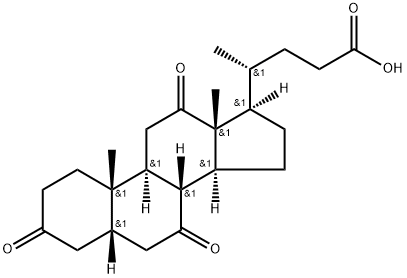
83-44-3
505 suppliers
$6.00/250mg

81-25-4
504 suppliers
$5.00/100mg

83-44-3
505 suppliers
$6.00/250mg

81-25-4
504 suppliers
$5.00/100mg
![4-[(5S,7S,8S,10S,13R,17R)-3,7,12-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid](/CAS/GIF/2955-27-3.gif)
2955-27-3
87 suppliers
$80.00/1mg