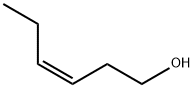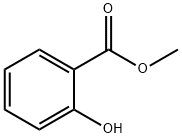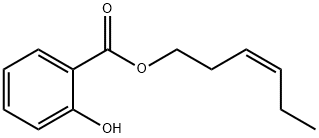
CIS-3-HEXENYL SALICYLATE synthesis
- Product Name:CIS-3-HEXENYL SALICYLATE
- CAS Number:65405-77-8
- Molecular formula:C13H16O3
- Molecular Weight:220.26
Yield:65405-77-8 89.2%
Reaction Conditions:
dibutyltin diacetate at 140 - 170; under 699.82 Torr; for 2 h;Product distribution / selectivity;
Steps:
3; 4
A four-necked glass flask were charged with 228.20 g (1.5 mol) of methyl salicylate and 166.49 g (1.66 mol) of cis-3-hexenol, and further 2.64 g (0.0075 mol: 0.5 mol % based on methyl salicylate) of di-n-butyl tin diacetate was added thereto under stirring. The temperature of the resultant mixture was gradually raised from 140° C. to 170° C. under an atmospheric pressure while distilling off methanol liberated out of the reaction system. After the temperature of the reaction solution reached 170° C., the pressure in the flask was reduced to 93.3 kPa at which the reaction solution was held under heating in a temperature range of from 160 to 170° C. Successively, while distilling off methanol liberated out of the reaction system, the contents of the flask were reacted with each other for about 2 h. The finally obtained reaction solution was subjected to gas chromatography for quantitative determination of cis-3-hexenyl salicylate. As a result, it was confirmed that the yield of the reaction product was 92.2%. Then, the thus obtained reaction solution was treated through ten-stage distillation columns. Specifically, first, 18.24 g of unreacted cis-3-hexenol was distilled off under a pressure of 1.3 kPa at a temperature of 87 to 130° C. and a reflux ratio of 1, and then 14.71 g of methyl salicylate was distilled off under a pressure of 1.3 kPa at a temperature of 130 to 159° C. and a reflux ratio of 1. Further, 25.52 g of initial distilled fraction containing residual methyl salicylate was distilled off under a pressure of 1.3 kPa at a temperature of 160° C. and a reflux ratio of 5. Next, the reaction solution was further subjected to distillation under a reduced pressure of 1.3 kPa at a temperature of 160 to 163° C. to obtain 258.88 g of cis-3-hexenyl salicylate. As a result, it was confirmed that the resultant distillation residue was in a liquid state and produced at a yield of 15.94 g (4.7% by weight based on the raw materials initially charged), and further the distillation residue exhibited a good handling property and was composed mainly of cis-3-hexenyl salicylate containing the tin catalyst. EXAMPLE 4 Into 14.50 g of the distillation residue obtained in the Example 3 were added only 228.58 g (1.5 mol) of methyl salicylate and 166.47 g (1.66 mol) of cis-3-hexenol, and the temperature of the resultant mixture was gradually raised from 140° C. to 170° C. under stirring while distilling off methanol liberated therefrom. After the temperature of the reaction solution reached 170° C., the pressure of the reaction system was reduced to 93.3 kPa at which the reaction solution was held under heating in a temperature range of from 160 to 170° C. Successively, while distilling off methanol liberated, the reaction solution was reacted for about 2 h. The finally obtained reaction solution was subjected to gas chromatography for quantitative determination of cis-3-hexenyl salicylate. As a result, it was confirmed that the yield of the reaction product was 89.2% exclusive of the amount of cis-3-hexenyl salicylate contained in the distillation residue.
References:
Kao Corporation US2006/58547, 2006, A1 Location in patent:Page/Page column 5