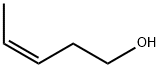
CIS-3-PENTEN-1-OL synthesis
- Product Name:CIS-3-PENTEN-1-OL
- CAS Number:764-38-5
- Molecular formula:C5H10O
- Molecular Weight:86.13
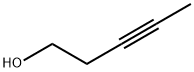
10229-10-4
87 suppliers
$17.00/1g

764-38-5
16 suppliers
inquiry
Yield: 94%
Reaction Conditions:
with quinoline;hydrogen;Lindlar catalyst in diethyl ether at 20; under 750.075 Torr; for 20 h;
Steps:
II.1.1.1
II. Preparation of the Substrates1. Preparation of (Z)-pent-3-enoic acid ((Z)-3) 1.1 (Z)-Pent-3-en-1-ol Lindlar catalyst (45 mg) was placed in a 250 ml Schlenk flask and degassed. Quinoline (780 mg, distilled under argon), diethyl ether (150 ml, abs.) and pent-3-yn-1-ol (2.74 ml, 2.5 g, 29.7 mmol) were added. The argon atmosphere was replaced by an H2 atmosphere. The hydrogenation was carried out at an H2 pressure of 1 bar at room temperature for 20 hours. The reaction mixture obtained was filtered through Celite and washed through with diethyl ether. The filtrate was freed of the solvent under reduced pressure. The residue was purified by distillation (140° C./atmospheric pressure). (Z)-Pent-3-en-1-ol was obtained as a colorless liquid in an amount of 2.4 g (yield: 94%). The content of (Z)-isomer in the product obtained was >96% according to GC analysis (GC: 6890N AGILENT TECHNOLOGIES; column: 24079 SUPELCO, Supelcowax 10, 30.0 m×0.25 mm×0.25 μm; 75° C. isothermal, He flow 0.7 ml/min; (E): 18.9 min., (Z): 19.3 min.).1H-NMR (400.1 MHz, CDCl3): δ=1.64 (d, J=5.6 Hz, 3H); 2.33 (pseudo-q, J=7.0 Hz, 2H); 3.63 (t, J=6.6 Hz, 2H); 5.35-5.45 (m, 1H); 5.57-5.67 ppm (m, 1H). 13C{1H}-NMR (100.6 MHz, CDCl3): δ=12.5 (s); 30.3 (s); 62.0 (s); 126.0 (s); 126.5 ppm (s); signals of the by-product ((E)-isomer): δ=17.4; 35.7; 61.9; 127.2; 127.5 ppm.
References:
BASF SE US2010/240896, 2010, A1 Location in patent:Page/Page column 8-9

4949-20-6
0 suppliers
inquiry

764-38-5
16 suppliers
inquiry
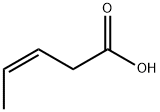
33698-87-2
6 suppliers
inquiry
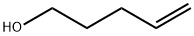
821-09-0
240 suppliers
$21.21/1gm:

764-38-5
16 suppliers
inquiry
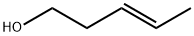
764-37-4
32 suppliers
inquiry
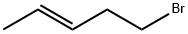
7515-62-0
1 suppliers
inquiry

764-38-5
16 suppliers
inquiry