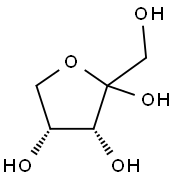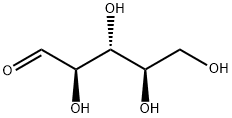
D-Ribose synthesis
- Product Name:D-Ribose
- CAS Number:50-69-1
- Molecular formula:C5H10O5
- Molecular Weight:150.13
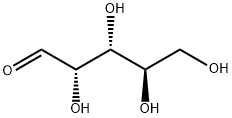
10323-20-3
429 suppliers
$10.00/10g

50-69-1
663 suppliers
$5.00/5g
Yield:-
Reaction Conditions:
with borax in water at 69.84; for 1 h;Kinetics;stereospecific reaction;pH-value;
Steps:
For the factorial design experiments, Sn-Beta (Si/Sn = 96) wasadded at a 100:1 sugar-metal molar ratio to a 5 wt% sugar solutionin a 5 ml thick-walled glass reactor containing a small magneticstir bar (typically ~40 mg of catalyst in 2 ml of sugar solution).Reactions were performed with D- and L-arabinose interchangeably with no difference in kinetics. Initial rate experiments weredone with larger volumes and higher sugar-metal ratios in orderto capture the low conversion data points. In order to determinethe initial rates at higher temperature ranges, an arabinose to Snratio of 1000 or 2000 was used. The thick-walled glass vials weresealed using a PTFE/silicone septa and metal crimp top, placed in atemperature-controlled oil bath, and removed periodically to takesamples. The glass reactors were quenched in ice and a small sam-ple volume was removed. Next, 100 l of the filtered sample and25 l of a 10 wt% mannitol solution were mixed in a vial. The sam-ples were analyzed by high-performance liquid chromatography(HPLC) on an Agilent 1260 system equipped with photodiode arrayultraviolet and evaporative light-scattering detectors. The reactionproducts were separated using a Bio-Rad Aminex HPX-87C columnheated to 353 K using deionized water (pH = 7) as the mobile phaseat a flow rate of 0.6 ml min-1. Ribulose concentrations were deter-mined using an ultraviolet detector at a wavelength 210 nm. At thiswavelength all other sugars had negligible ultraviolet signals. Forall ultraviolet-inactive carbohydrates, the analysis was performedusing the evaporative light scattering detector. Samples were runin duplicate on the HPLC and the column was regenerated dailywith 0.1 M Ca(NO3)2. Ketoses showed the strongest tendency toshift retention time in the column. Fractionation and NMR wereused to check peak purity.
References:
Gunther, William R.;Duong, Quynh;Román-Leshkov, Yuriy [Journal of Molecular Catalysis A: Chemical,2013,vol. 379,p. 294 - 302]
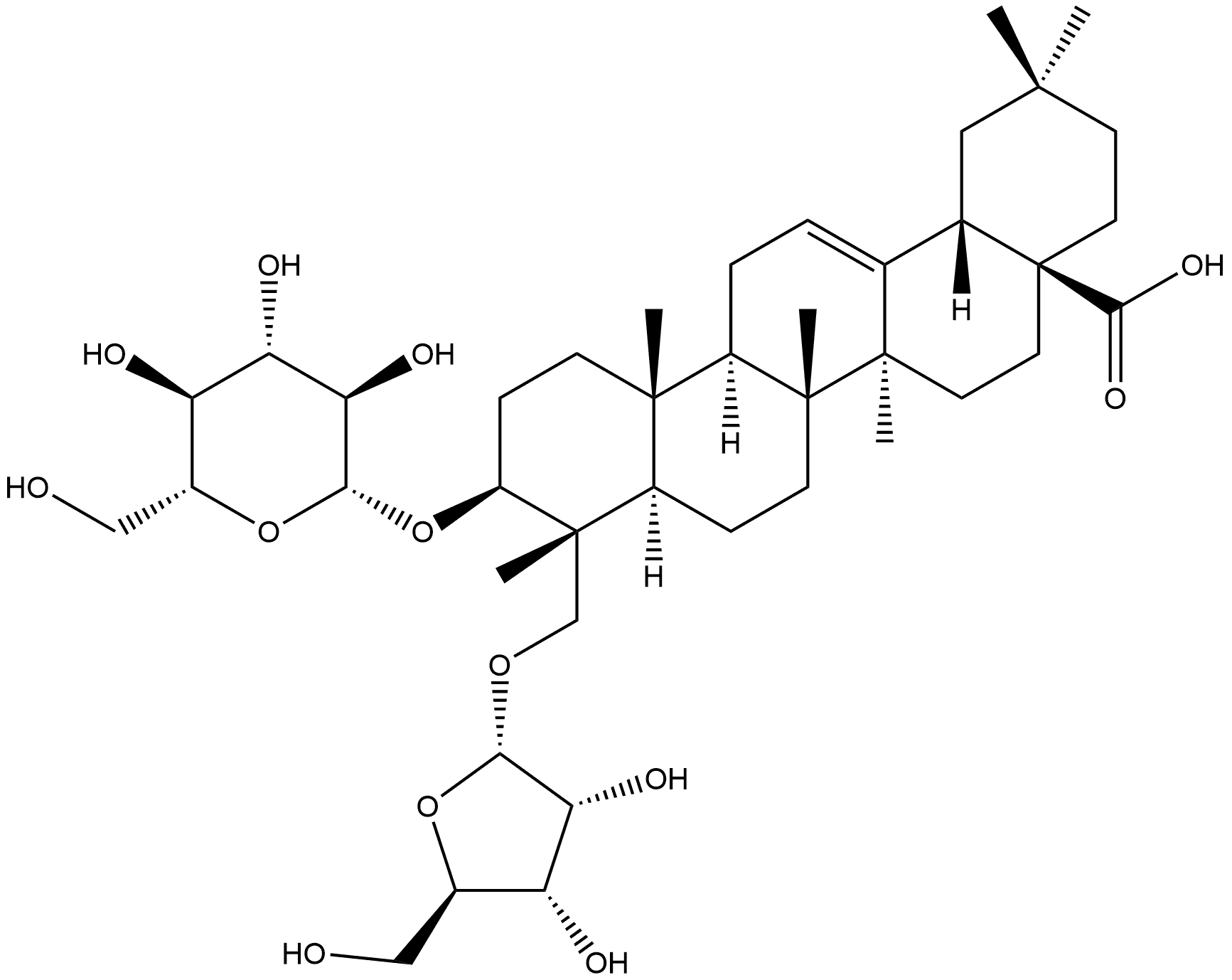
1500092-25-0
0 suppliers
inquiry

50-69-1
663 suppliers
$5.00/5g
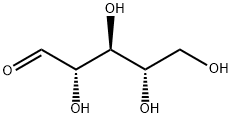
24259-59-4
350 suppliers
$5.00/1g
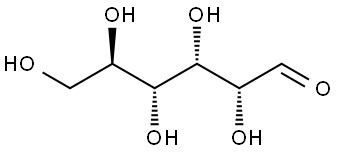
50-99-7
962 suppliers
$6.00/25g
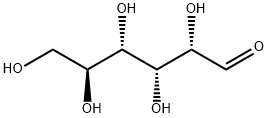
921-60-8
252 suppliers
$32.00/250mg
51246-99-2
0 suppliers
inquiry

50-69-1
663 suppliers
$5.00/5g