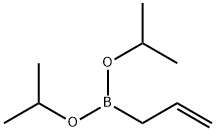
Diisopropyl Allylboronate synthesis
- Product Name:Diisopropyl Allylboronate
- CAS Number:51851-79-7
- Molecular formula:C9H19BO2
- Molecular Weight:170.06

5419-55-6
369 suppliers
$12.00/5g

1730-25-2
195 suppliers
$53.20/100ml

51851-79-7
33 suppliers
$75.00/100mg
Yield: 75%
Reaction Conditions:
in diethyl ether at -78 - 0; for 4 h;Inert atmosphere;
Steps:
1.1 Step 1: Synthesis of 1C
A 3 L three-neck round-bottom flask equipped with addition funnel, thermometer and mechanical stirrer was charged with triisopropyl borate 1A (153.5 g, 0.816 mol) and ether (1 L) under nitrogen. The flask was cooled to -78 oC and allylmagnesium bromide 1B (800 mL, 0.80 mol, 1M in ether) was added via addition funnel over 1 hour with vigorous stirring. The resulting reaction mixture was slowly warmed up to 0 oC over 2 hours, and allowed to stay at 0 oC for another 1 hour. The reaction mixture was then cooled below -70 oC and hydrogen chloride solution (408 mL, 0.816 mol, 2.0 M in ether) was added via the addition funnel in 25 min. After addition was completed, the bath was removed and the mixture was slowly warmed up to room temperature in 1.5 hours. After reaching room temperature, the stirring was stopped and the mixture was allowed to settle down. The top clear solution was decanted and the remaining grey solid was washed with ether (500 mL X 2) and filtered through Celite. The ether solution was concentrated and the top layer was decanted from bottom viscous orange layer. The top layer was distilled under reduced pressure (~ 10 mmHg) to give a clear colorless liquid 1C (103 g, 75% yield).
References:
REMPEX PHARMACEUTICALS, INC.;HECKER, Scott;REDDY, Raja, K.;GLINKA, Tomasz WO2016/81297, 2016, A1 Location in patent:Paragraph 0149; 0150