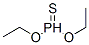
DISULFOTON synthesis
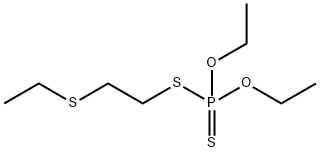
- Product Name:DISULFOTON
- CAS Number:298-04-4
- Molecular formula:C8H19O2PS3
- Molecular Weight:274.4
Yield:-
Reaction Conditions:
with sodium hydroxide in water; pH=6 at 70; for 4 h;
Steps:
1; 2 Example 1; Inventive Method
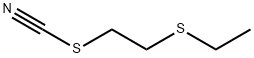
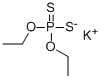
The composition containing O,O-diethyl dithiophosphoric acid sodium salt was allowed to separate into an aqueous portion and an organic portion. The aqueous portion was collected. About 250 ml of the aqueous portion was placed in a 500 ml four-neck round bottom flask and heated to about 70° C. under nitrogen. About 89.6 g of β-chloroethyl ethyl sulfide (93% purity) was added over about 30 minutes. The resulting mixture was stirred for about 3.5 hours. The pH was maintained at about 6 with 50% by weight NaOH. The mixture was filtered hot under vacuum. The upper organic phase was collected and washed with about 173 g of water. The product was dried by rotary evaporation at about 65° C. under aspirator vacuum for about 3 hours. About 0.67 g of O,O-diethyl S-[2-(ethylthio)ethyl]phosphorodithioate (96.9% purity) was obtained.Example 2 Comparative Method [0053] A composition containing O,O-diethyl dithiophosphoric acid sodium salt was obtained by mixing O,O-diethyl dithiophosphoric acid with an about 25% by weight of NaOH solution in the presence of toluene. [0054] About 250 g of the composition containing O,O-diethyl dithiophosphoric acid sodium salt was placed in a separatory funnel, and about 5.1 g of toluene was added with agitation. The mixture was filtered before separation and the lower aqueous phase was collected. [0055] About 250 ml of the aqueous phase was placed in a 500 ml four-neck round bottom flask and heated to about 70° C. under nitrogen. About 89.6 g of β-chloroethyl ethyl sulfide (93% purity) was added over about 30 minutes. The resulting mixture was stirred for about 3.5 hours. The pH was maintained at about 6 with 50% by weight NaOH. The mixture was filtered hot under vacuum. The upper organic phase was collected and washed with about 173 g of water. The final product was dried by rotary evaporation at about 65° C. under aspirator vacuum for about 3 hours. About 0.71 g of O,O-diethyl S-[2-(ethylthio)ethyl]phosphorodithioate (97.2% purity) was obtained.
References:
Prasad, Vidyanatha A.;Ingalls, Robert D.;Slahck, Stephen C.;Tusa, Christopher M. US2004/176629, 2004, A1 Location in patent:Page 4
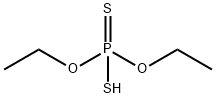
298-06-6
151 suppliers
$62.50/5 g
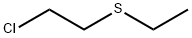
693-07-2
123 suppliers
$35.00/1 g

298-04-4
2 suppliers
$54.00/250mg