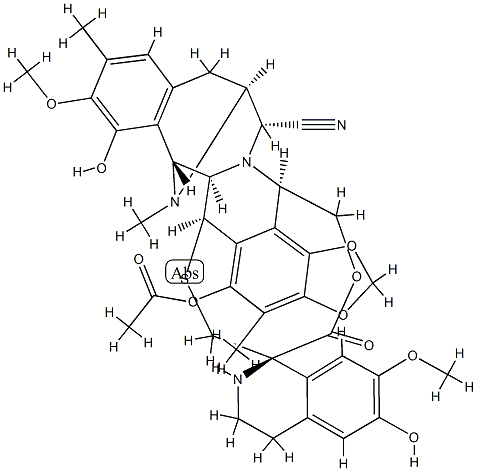
Ecteinascidin 770 synthesis
- Product Name:Ecteinascidin 770
- CAS Number:114899-80-8
- Molecular formula:C40H42N4O10S
- Molecular Weight:770.85
Yield: 33.4% , 27.8%
Reaction Conditions:
Stage #1:triallyl-ecteinascidin 770 with bis-triphenylphosphine-palladium(II) chloride;tri-n-butyl-tin hydride;acetic acid in tetrahydrofuran at 25; for 6 h;
Stage #2: with sodium hydrogencarbonate in tetrahydrofuran;water
Steps:
5.1.2. Deallylation of 4c: 2'-N-allyl Et 770 4d
Tributyltin hydride (0.16 mL, 0.61 mmol) was added dropwise over 10 min to a vigorously stirred solution of 4c (32.9 mg, 37.0 μmol), (Ph3P)2PdCl2 (15.6 mg, 22.2 μmol), and AcOH (79.4 μL, 1.39 mmol) in THF (10 mL) at 25 °C, and the mixture was stirred for 6 h at 25 °C. The mixture was diluted with water (10 mL), made alkaline with 5% aqueous NaHCO3, and extracted with chloroform (30 mL × 3). The combined extracts were washed with 5% aqueous NaHCO3, dried, and concentrated in vacuo to give a residue. Chromatography on a silica gel column with hexane-ethyl acetate (5:1) to gave 1b (9.5 mg, 33.4%) as a colorless solid, whose spectral data were in complete agreement with those of the authentic sample. Further elution with hexane-ethyl acetate (2:1) afforded 2'-N-Allyl Et 770 (4d: 8.3 mg, 27.8%). [α]D22 -80.1 (c 0.25, CHCl3); IR (KBr) 3447br, 2930, 2250w, 1749, 1508, 1458, 1375, 1234, 1196, 1161, 1107, 1086, 1005, 914 cm-1; 1H NMR (CDCl3, 500 MHz) δ 6.47 (1H, s, 15-H), 6.45 (1H, s, 5'-H), 6.22 (1H, s, 8'-H), 6.08 (1H, d, J = 1.2 Hz, OCHO), 5.97 (1H, d, J = 1.2 Hz, OCHO), 5.69 (1H, s, 18-OH), 5.57 (1H, ddt, J = 17.0, 10.2, 6.3 Hz, NCH2CHCH2), 5.42 (1H, br s, 6'-OH), 5.11 (1H, dd, J = 17.0, 1.7 Hz, NCH2CHCH2), 5.02 (1H, dd, J = 10.2, 1.7 Hz, NCH2CHCH2), 4.96 (1H, d, J = 11.4 Hz, 22-H), 4.62 (1H, br s, 4-H), 4.37 (1H, d, J = 2.3 Hz, 1-H), 4.26 (1H, dd, J = 5.1, 1.2 Hz, 11-H), 4.11 (1H, d, J = 2.9 Hz, 21-H), 3.91 (1H, dd, J = 11.4, 2.9 Hz, 22-H), 3.76 (3H, s, 17-OCH3), 3.56 (3H, s, 7'-OCH3), 3.53 (1H, d, J = 5.1 Hz, 3-H), 3.42 (1H, br d, J = 9.0 Hz, 13-H), 3.08 (2H, br d, NCH2CH), 2.95 (1H, dd, J = 17.0, 9.1 Hz, 14-Hα), 2.94 (1H, m. 3'-H), 2.80 (1H, d, J = 17.0 Hz, 14-Hβ), 2.74 (1H, dt, J = 12.5, 4.5 Hz, 3'-H), 2.56 (1H, ddd, J = 15.8, 10.2, 5.1 Hz, 4'-H), 2.56 (1H, br, 12'-H), 2.49 (1H, m, 12'-H), 2.34 (1H, dt, J = 15.8, 3.9 Hz, 4'-H), 2.30 (3H, s, OCOCH3), 2.25 (3H, s, 16-CH3), 2.11 (3H, s, NCH3), 2.03 (3H, s, 6-CH3); 13C NMR (CDCl3, 125 MHz) δ 169.6 (1'-CO), 168.4 (5-OCO), 147.3 (C-18), 145.5 (C-7), 144.5 (C-6'), 144.1 (C-7'), 142.9 (C-17), 141.8 (C-5), 140.6 (C-8), 138.1 (NCH2CHCH2), 130.7 (C-20), 130.2 (C-10'), 129.4 (C-16), 127.4 (C-9'), 121.6 (C-10), 121.2 (C-15), 118.4 (C-19), 118.3 (21-CN), 115.4 (NCH2CHCH2), 114.5 (C-9), 113.9 (C-5'), 112.5 (C-6), 110.9 (C-8'), 102.0 (OCH2O), 71.1 (C-1'), 61.1 (C-3), 60.7 (C-22), 60.4 (C-1), 60.1 (17-OCH3), 60.0 (C-21), 55.4 (7'-OCH3), 54.9 (C-11), 54.7 (C-13), 54.2 (NCH2CH), 43.7 (C-3'), 43.0 (C-4), 41.8 (NCH3), 39.7(C-12'), 27.8 (C-4'), 24.9 (C-14), 20.3 (COCH3), 16.1 (16-CH3), 9.7 (6-CH3); FABMS m/z 811 (MH+); HRFABMS m/z 811.3011 (MH+, calcd for C43H47N4O10S, 811.3013), CD Δε nm (c 12.3 μM, methanol, 32 °C) -29.4 (210), -66.7 (218), 0 (244), 9.6 (253), 0 (270), -13.8 (286), 0 (320).
References:
Saktrakulkla, Panithi;Toriumi, Satoru;Tsujimoto, Mitsuhiro;Patarapanich, Chamnan;Suwanborirux, Khanit;Saito, Naoki [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 15,p. 4421 - 4436] Location in patent:experimental part
645-33-0
155 suppliers
$17.00/10mg
308359-33-3
12 suppliers
inquiry

114899-80-8
40 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

114899-80-8
40 suppliers
inquiry
111726-43-3
17 suppliers
inquiry

114899-80-8
40 suppliers
inquiry