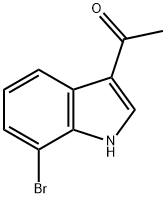
Ethanone, 1-(7-broMo-1H-indol-3-yl)- synthesis
- Product Name:Ethanone, 1-(7-broMo-1H-indol-3-yl)-
- CAS Number:944086-09-3
- Molecular formula:C10H8BrNO
- Molecular Weight:238.08
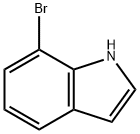
51417-51-7
362 suppliers
$12.00/1g

75-36-5
560 suppliers
$17.92/100G

944086-09-3
48 suppliers
$66.00/100mg
Yield:944086-09-3 93%
Reaction Conditions:
Stage #1: 7-bromo-1H-indolewith diethylaluminium chloride in dichloromethane at 0; for 0.416667 h;
Stage #2: acetyl chloride in dichloromethane at 0; for 1 - 3 h;
Steps:
3
Typical procedure 3 (See Scheme 2) (TP3):1 -(7-bromo-l -(3-chloroproρyO-l H-indol-3-yDethanone[0216] To a solution of 7-bromo-l //-indole (442 mg, 2.25 mmol) in dry CH2Cl2 (10 mL) at 0 0C was added Et2AlCl (3.4 mL, 1.0 M, 3.4 mmol) dropwise. The mixture was stirred at 0 0C for 25 min. A solution of AcCl (0.24 mL, 3.36 mmol) in CH2Cl2 (5 mL) was added dropwise and the mixture was stirred at 0 0C until TLC showed complete conversion of the indole (1-3 h). Saturated aqueous NaHCO3 (10 mL) was added slowly and the mixture was allowed to reach room temperature. The mixture was diluted with CH2Cb (60 mL) and the mixture was cautiously acidified to pH 4-5 with 2 M HCl (approx. 10 mL) to facilitate phase separation. The aqueous layer was extracted with CH2Cl2 (2x 10 mL) and the combined organic layers where washed with saturated aqueous NaHCθ3 and evaporated to dryness to give the acetylated compound (496 mg, 93%). This acetyl ated crude product was dissolved in CH3CN (10 mL). To this solution was added Cs2CO3 (1.55 g, 4.76 mmol) and 1 - chloro-3-iodopropane (0.70 mL, 6.52 mmol) and the mixture was stirred at 50 0C overnight. The suspension was diluted with CH2Cl2 (70 mL), filtered and adsorbed onto celite. After purification by flash chromatography (heptanes - > heptanes: EtOAc 3:2) the title product was obtained (61 1 mg, 86% over two steps.
References:
WO2007/79239,2007,A2 Location in patent:Page/Page column 75-76

51417-51-7
362 suppliers
$12.00/1g

108-24-7
5 suppliers
$14.00/250ML

944086-09-3
48 suppliers
$66.00/100mg