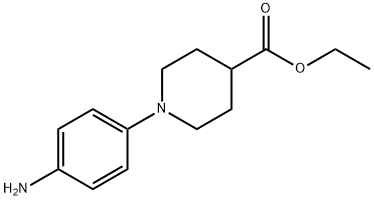
ETHYL 1-(4-AMINOPHENYL)-4-PIPERIDINECARBOXYLATE synthesis
- Product Name:ETHYL 1-(4-AMINOPHENYL)-4-PIPERIDINECARBOXYLATE
- CAS Number:439095-52-0
- Molecular formula:C14H20N2O2
- Molecular Weight:248.32
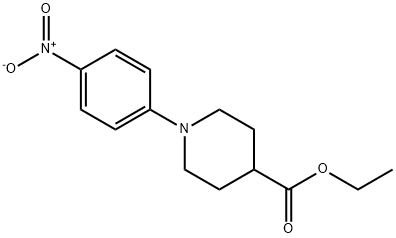
216985-30-7
18 suppliers
inquiry

439095-52-0
51 suppliers
$45.00/50mg
Yield:439095-52-0 97%
Reaction Conditions:
with iron;ammonium chloride in methanol at 80; for 5 h;
Steps:
18.B B. Ethyl 1-(4-aminophenyl)piperidine-4-carboxylate,18b
[0567] To a solution ofcompound 18a (15.0 g, 51.2 mmol)in MeOH (100 mL) was added Fe (9.06 g, 162 mmol) andsaturated NH4Cl solution (100 mL). The resulting solutionwas stirred at 80° C. for 5 h. After cooling to rt, the mixturewas extracted with EtOAc (200 mL). The organic layer waswashed with brine, dried over Na2 S04 and concentratedunder reduced pressure. The residue was purified by flashcolunm chromatography on silica gel, eluting with EtOAc/petroleum ether (1 :5 v/v) to obtain compound 18b as a blacksolid (13.0 g, 97% yield). Mass Spectrum (LCMS, ESI pos.):Calcd. for C14H20N2 0 2 : 249.2 (M+H). Found 249.0.
References:
US2014/364414,2014,A1 Location in patent:Paragraph 0563; 0566-0567
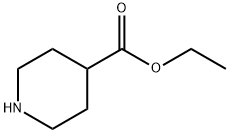
1126-09-6
491 suppliers
$15.40/25g

439095-52-0
51 suppliers
$45.00/50mg
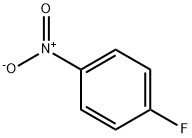
350-46-9
514 suppliers
$10.60/1gm:

439095-52-0
51 suppliers
$45.00/50mg