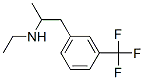
Fenfluramine hydrochloride synthesis
- Product Name:Fenfluramine hydrochloride
- CAS Number:404-82-0
- Molecular formula:C12H17ClF3N
- Molecular Weight:267.72
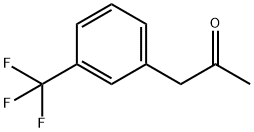
21906-39-8
192 suppliers
$8.00/1g
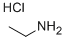
557-66-4
277 suppliers
$28.00/25g

404-82-0
73 suppliers
$24.00/1mg
Yield:404-82-0 148 g
Reaction Conditions:
Stage #1: ethanamine hydrochloridewith sodium hydroxide in methanol at 0 - 15; for 0.5 h;
Stage #2: 3-(trifluoromethyl)phenylacetone in methanol at 0 - 5; for 0.5 h;Further stages;Reagent/catalyst;Temperature;
Steps:
4 Example-4: Preparation of compound of formula-la.
Around bottom flask was charged with sodium hydroxide (112 g ), methanol (528 mL) stirred at 0-5 °C for 15 min. Ethylamine hydrochloride (225 g) was added to the above reaction mixture at same temperature and stirred for 30 min. A compound of formula-4 (160 g ) was added to the reaction mass at 0-5 °C and stirred for 30 min. Sodium borohydride (28.8 g) was added portion wise to the above reaction mixture keeping the temperature below 10 °C and stirred for 30 min. The reaction mixture was warmed to 20-25°C, stirred for 5 hours. Methanol was distilled off under reduced pressure below 40°C, added water (650 mL), dichloromethane (350 mL) stirred for 15 min. The organic layer was separated, washed with water (200 mL x 3) and the organic layer was evaporated to get Fenfluramine free base as colorless oil (160 g). The obtained Fenfluramine crude compound (160 g) was diluted with ethyl acetate (1200 mL), adjusted the pH upto 1.0 by using isopropyl alcohol hydrochloride (245 mL) at 0-5 °C and stirred for 30 min. The reaction mixture was warmed to 25-30 °C and stirred for 1 hr, the obtained solid was filtered and dried to obtain the title compound. (0058) Yield : 148 g . (0059) Purity by HPLC: >99.9 %
References:
WO2021/117057,2021,A1 Location in patent:Page/Page column 10-12

713-45-1
47 suppliers
$30.00/250mg
75-04-7
371 suppliers
$10.00/20mg

404-82-0
73 suppliers
$24.00/1mg
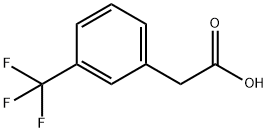
351-35-9
221 suppliers
$9.00/5g

404-82-0
73 suppliers
$24.00/1mg
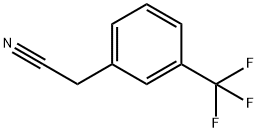
2338-76-3
272 suppliers
$7.00/5g

404-82-0
73 suppliers
$24.00/1mg

21906-39-8
192 suppliers
$8.00/1g

404-82-0
73 suppliers
$24.00/1mg