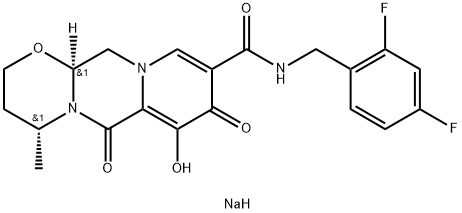
Dolutegravir sodium synthesis
- Product Name:Dolutegravir sodium
- CAS Number:1051375-19-9
- Molecular formula:C20H20F2N3NaO5
- Molecular Weight:443.38
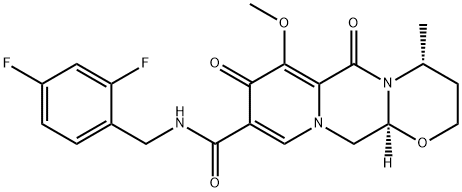
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide](/CAS/20150408/GIF/1206102-11-5.gif)
1206102-11-5
84 suppliers
inquiry

1051375-19-9
216 suppliers
inquiry
Yield:1051375-19-9 98.9%
Reaction Conditions:
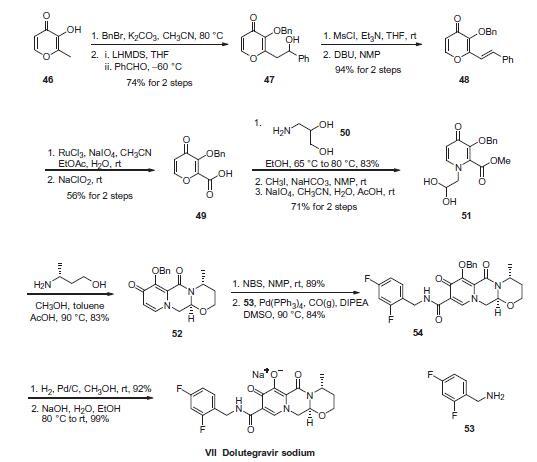
Stage #1: (4R,12aS)-7-(benzyloxy)-N-(2,4-difluorobenzyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1′,2’:4,5]-pyrazino[2,1-b][1,3]oxazine-9-carboxamidewith propylene glycol;palladium 10% on activated carbon;hydrogen at 50;
Stage #2: with sodium t-butanolate at 30 - 70;Temperature;
Steps:
4b Example 4 (General Procedure) Examples 4a, 4b, 4d and 4e: 1 .00 g (1 .96 mmol)
General procedure: Example 4 (General Procedure) Examples 4a, 4b, 4d and 4e: 1 .00 g (1 .96 mmol) DTG-OBn was suspended in 10 mL racemic 1 ,2-PG and 5 mL of a co-solvent. The flask was flushed with argon and then charged with 42 mg of 10% palladium on activated charcoal (50% wet, 0.20 mmol, 0.01 eq.). The resulting mixture was heated to 50 °C and stirred under hydrogen atmosphere until completion. The flask was flushed with argon and the warm mixture was filtered over glass fiber filter. The filtrate was charged into a clean flask and heated to the desired temperature (see Table below). A solution of 192 mg (1 .96 mmol, 1 .0 eq.) sodium tert-butylate in 2 ml. racemic 1 ,2-PG was added to the mixture, under vigorous stirring. After completion of the addition, a clear yellow solution was obtained. A solid started to precipitate within 5 min. After stirring for 5-15 min, the heating of the oil bath was turned off and after 90 min (T (oil bath) = approx. 30°C), the oil bath was removed, the mixture was stirred for another 30 min at RT and then filtered. The isolated solid was washed with acetone and dried at 50°C under vacuum (10 mbar) for different times (see table) yielding Dolutegravir sodium salt (1 :1 ) 1 ,2-propylene glycol solvate as off-white solid.
References:
WO2017/46131,2017,A1 Location in patent:Page/Page column 30; 31
1335210-35-9
78 suppliers
inquiry

1051375-19-9
216 suppliers
inquiry
![(S)-7-hydroxy-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxylic acid](/CAS/20150408/GIF/1246616-73-8.gif)
1246616-73-8
58 suppliers
$160.00/1mg
72235-52-0
401 suppliers
$12.00/5g

1051375-19-9
216 suppliers
inquiry
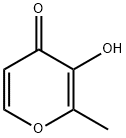
118-71-8
599 suppliers
$5.00/25mg

1051375-19-9
216 suppliers
inquiry
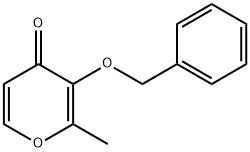
61049-69-2
83 suppliers
$68.00/25mg

1051375-19-9
216 suppliers
inquiry