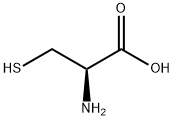
L-Cysteine synthesis
- Product Name:L-Cysteine
- CAS Number:52-90-4
- Molecular formula:C3H7NO2S
- Molecular Weight:121.16
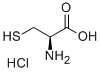
52-89-1
642 suppliers
$5.00/50mg

52-90-4
886 suppliers
$6.00/10g
Yield:52-90-4 92%
Reaction Conditions:
in acetone; for 1.5 h;Heating / reflux;
Steps:
90
L-cysteine hydrochloride monohydrate (100 g, 0.569 mol) was refluxed in dry acetone (1.5 L) under dry nitrogen for 1.5 hours. The white precipitate was collected by filtration and refluxed a second time in dry acetone. Again the white solid was collected to yield 103.6 g of pure product (92 % yield).
References:
WO2007/1926,2007,A2 Location in patent:Page/Page column 257

112839-94-8
0 suppliers
inquiry

52-90-4
886 suppliers
$6.00/10g
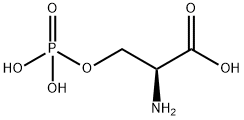
407-41-0
180 suppliers
$9.00/100mg

52-90-4
886 suppliers
$6.00/10g
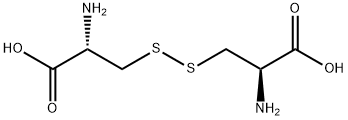
56-89-3
829 suppliers
$5.00/10g

52-90-4
886 suppliers
$6.00/10g
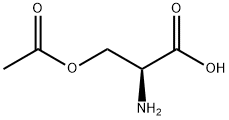
5147-00-2
85 suppliers
$37.00/100mg

52-90-4
886 suppliers
$6.00/10g