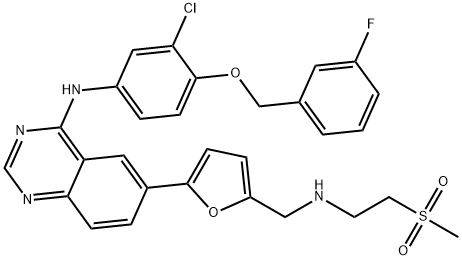
Lapatinib synthesis
- Product Name:Lapatinib
- CAS Number:231277-92-2
- Molecular formula:C29H26ClFN4O4S
- Molecular Weight:581.06

Zhang, Yaling; Zhang, Ying; Liu, Juan; Chen, Li; Zhao, Lijun; Li, Baolin; Wang, Wei. Synthesis and in vitro biological evaluation of novel quinazoline derivatives. Bioorganic & Medicinal Chemistry Letters. Volume 27. Issue 7. Pages 1584-1587. 2017

1227853-06-6
4 suppliers
inquiry

231277-92-2
473 suppliers
$20.00/50mg
Yield:-
Reaction Conditions:
Stage #1: N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(5-((2-(methylsulfonyl)ethylimino)methyl)furan-2-yl)quinazolin-4-aminewith methanol;sodium tetrahydroborate in tetrahydrofuran at 0 - 15;
Stage #2: with water in tetrahydrofuran;
Steps:
2.v
(v) Preparation of N{3-chloro-4-[(3-fluorobenzyIoxy]phenyl}-6-[5-({[2- methanesuIphonyl)ethyl]amino}methyl)-2-furyI]-4-quinazolinamine (or) Lapatinib base (1)Into a two liter four-necked round bottomed flask, 40OmL of tetrahydrofuran, 40 g of imine obtained from the previous step-(iv), 400 ml of methanol were charged under stirring. The reaction mass was cooled to 0 to 5° C and 7.0 g of sodium borohydride was added in lots and the reaction mass was maintained for about 4 hrs at 10 to 15 0C. The completion of the reaction was monitored by HPLC. To this reaction mass 800 ml of water was added and the product was extracted into ethylacetate. The organic layer was separated and the solvent distilled off completely under vacuum. The solvent was distilled off completely under vacuum. The residue was cooled to 25-35°C and 80 mL of ethylacetate was added, stirred for 2 hrs, filtered and dried under vacuum at 40-450C to get 30.5 g (75% on theory) of crude Lapatinib base. Purity : 90% by HPLC The purity of the above product was enhanced by adopting the following procedure. Purification:Into a two liter four-necked round-bottomed flask, 120OmL of methanol, 30.0 g of Lapatinib crude base obtained as above were charged under stirring. The mass was maintained at 60- 65° C for 30-45 minutes and filtered the undissolved material. The filtrate was distilled off completely under vacuum. The mass was cooled to 25-350C. To the residue 60 mL of methanol was added, stirred for 2 hrs, filtered and dried the product under vacuum at 40-450C to get 28 g of pure Lapatinib base. Purity: 99.5% by HPLC Melting point range: 95-98° C (Peak maximum by DSC) IR (KBr, cm"1): 3485.8, 3303.7, 3060.2, 2924.4, 2814.6, 1921.8, 1592.3, 1573.6, 1525.8, 1490.4, 1457.4, 1422.1, 1385.8, 1365.8, 1337.8, 1319.3, 1288.9, 1268.0, 1215.5, 1133.7, 1060.6, 1029.9, 941.1, 849.3, 779.3, 747.2, 682.0, 552.1, 520.3, 477.7. 2Θ values by XRPD: 11.17, 11.59, 12.30, 12.80, 14.84, 16.15, 16.52, 17.71, 18.88, 20.89, 21.63, 22.37, 22.77, 23.17, 23.80, 24.91, 25.68, 26.61, 28.07, 29.39, 29.87, 30.60, 31.35, 32.29, 34.42, 36.77, 39.41, and 41.31.
References:
WO2010/61400,2010,A1 Location in patent:Page/Page column 16-17

388082-76-6
20 suppliers
$333.00/1g
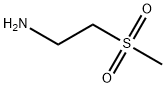
49773-20-8
58 suppliers
$41.00/250mg

231277-92-2
473 suppliers
$20.00/50mg

1618648-59-1
2 suppliers
inquiry

231277-92-2
473 suppliers
$20.00/50mg

1334953-75-1
13 suppliers
inquiry
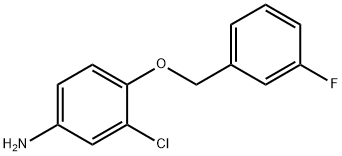
202197-26-0
301 suppliers
$6.00/1g

231277-92-2
473 suppliers
$20.00/50mg








