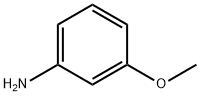
m-Anisidine synthesis
- Product Name:m-Anisidine
- CAS Number:536-90-3
- Molecular formula:C7H9NO
- Molecular Weight:123.15
A mixture of 35 g. (0.23 mole) of m-nitroanisole (p. 213), 110 ml. of methanol, and 7.5 ml. of concentrated hydrochloric acid is stirred and heated to boiling. Forty-two grams (0.75 gram atom) of iron filings is added in small portions over a 1-hour period, and refluxing and stirring are continued for 5 additional hours. The mixture is made strongly alkaline with sodium hydroxide and steam-distilled, the methanol which first distils over being collected separately. The remainder of the distillate is extracted with ether; the ethereal solu-tion is dried over anhydrous sodium sulfate and distilled. m-Anisidine (23.2 g. or 80%) is collected at 125°/13 mm.
Reference: J. Chem. Soc, 1934, 1420; J. Chem. Soc, 127, 494 (1925).
4294-95-5
238 suppliers
$14.00/1g

536-90-3
293 suppliers
$10.00/5g
Yield:536-90-3 91%
Reaction Conditions:
with Imidazole hydrochloride in neat (no solvent) at 150; for 3 h;Green chemistry;
Steps:
2.1 Typical general procedures for the synthesis of desired products (2a-2o, except for 2a’, 2b’ 2i, 2j, 2m and 2n’).
General procedure: A round-bottom flask was charged with nitrogen-containing (hetero)aromatic carboxylic acids 1 (0.5 g, 1.0 equiv.) and imidazole hydrochloride (1.5 equiv.). The mixture was stirred at 150 °C for 3 h. The reaction was monitored by TLC (petroleum ether/ethyl acetate 5:1). When the reaction reached completion, the reaction mixture was allowed to cool, and then distilled water (25ml) was added. The aqueous phase was extracted with ethyl acetate (3 × 20 ml), combined with the organic phase, dried over anhydrous Na2SO4, and concentrated in vacuum with silica gel added. The residue was separated by chromatography (petroleum ether/ethyl acetate 5:1 AR) to obtain the desired products.
References:
Wang, Yin;Xu, Ping;Li, Yanwu;Wu, Yue;Liao, Siwei;Huang, Xin;Zhang, Xiuyu;Yuan, Jianyong [Tetrahedron Letters,2022,vol. 102,art. no. 153941] Location in patent:supporting information
10365-98-7
405 suppliers
$9.00/10g

536-90-3
293 suppliers
$10.00/5g
766-85-8
190 suppliers
$22.00/5g

536-90-3
293 suppliers
$10.00/5g
555-03-3
3 suppliers
$25.00/1g

536-90-3
293 suppliers
$10.00/5g
2398-37-0
566 suppliers
$8.00/10g

536-90-3
293 suppliers
$10.00/5g