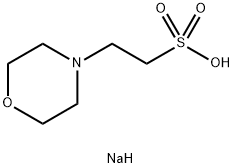
MES sodium salt synthesis
- Product Name:MES sodium salt
- CAS Number:71119-23-8
- Molecular formula:C6H14NNaO4S
- Molecular Weight:219.23
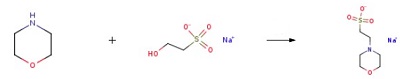

110-91-8
673 suppliers
$9.00/1g
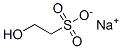
1562-00-1
384 suppliers
$5.00/25g

71119-23-8
300 suppliers
$16.80/25G
Yield:71119-23-8 44.2%
Reaction Conditions:
sodium hydroxide in water at 200; under 3345.86 - 8517.48 Torr; for 2 - 4 h;Product distribution / selectivity;Autoclave;
Steps:
B.12; C.13; D.11
Using the reaction vessel described in Procedure A, the amine starting material, aqueous sodium 2-hydroxyethanesulfonate, and aqueous 50 percent sodium hydroxide were charged to the autoclave. With the isolation valve closed, the stirred reaction mixture was rapidly heated to the target reaction temperature and held at that temperature for the times specified in Example 12. The isolation valve was then carefully opened and water vapor removed from the mixture until the targeted amount of distillate had been removed. An analysis of the collected aqueous distillate was performed by UV spectroscopy to determine the amount of amine which co-distilled with the water. The isolation valve was then closed and the reaction mixture further heated to a target temperature at the target temperature for an additional 2 hours. The reaction mixture was then cooled and diluted as described in Procedure A. Procedure C A magnetically-stirred 70-mL fluoropolymer-lined steel autoclave was fitted with an internal thermocouple capable of determining the temperature of the liquid phase contained in the vessel, a pressure gauge, a pressure-relief valve, and a vent valve. The amine starting material, anhydrous or aqueous sodium 2-hydroxyethanesulfonate, and anhydrous or aqueous sodium hydroxide were charged to the open autoclave, which was then assembled and immersed in an electrically heated oil bath. With the vent valve closed, the stirred reaction mixture was rapidly heated to the reaction temperature and held at that temperature for the times specified in Examples 9, 10, and 13 below. The reaction mixture was then cooled to ambient temperature, the reactor opened, and the reaction product taken up in sufficient water to dissolve all solids. Procedure D The reactor assembly described in Procedure C was connected to an overhead condenser and receiver assembly. The amine starting material, anhydrous or aqueous sodium 2-hydroxyethanesulfonate, and anhydrous or aqueous sodium hydroxide were charged to the open autoclave, which was then assembled and immersed in an electrically heated oil bath. With the vent valve closed, the stirred reaction mixture was rapidly heated to the reaction temperature and held at that temperature for the times specified in Example 11 below. A fixed portion of water was then removed as steam in a manner similar to Procedure B. The reactor was further heated as indicated below, then cooled to ambient temperature. The reactor was then opened, and the reaction product taken up in sufficient water to dissolve all solids.
References:
US2006/89509,2006,A1 Location in patent:Page/Page column 4; 6