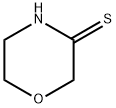
morpholine-3-thione synthesis
- Product Name:morpholine-3-thione
- CAS Number:21009-57-4
- Molecular formula:C4H7NOS
- Molecular Weight:117.17
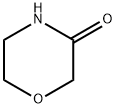
109-11-5
261 suppliers
$5.00/1g

21009-57-4
4 suppliers
inquiry
Yield:21009-57-4 72.2%
Reaction Conditions:
with Lawessons reagent in tetrahydrofuran; for 1.5 h;Heating / reflux;
Steps:
7.2
Step 2: MorPholin-3-thione; A mixture of morpholin-3-one (4.7 g) and Lawesson's reagent (10.3 g) in dry THF (94 mL) was heated to reflux for 1.5 h. The reaction mixture was cooled to room temperature and the reaction solvent was removed in vacuo. The residue was applied to silica gel column chromatography and eluted with CHCI3-methanol (50 : 1) to obtain a yellow solid. Recrystallization of the crude product from hexane-ethyl acetate gave the title (4.0 g, 72.2%) as yellow powder. 1H NMR (CDCI3) 8 3.45 (t, 2H, J = 5.1 Hz), 3.91 (t, 2H, J = 5.1 Hz), 4.55 (s, 2H).
References:
WO2003/93279,2003,A1 Location in patent:Page/Page column 64