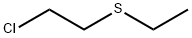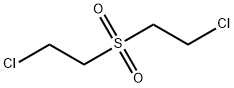
MUSTARDSULPHONE synthesis
- Product Name:MUSTARDSULPHONE
- CAS Number:471-03-4
- Molecular formula:C4H8Cl2O2S
- Molecular Weight:191.08
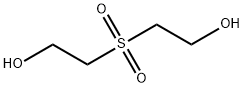
2580-77-0
114 suppliers
$10.00/1g

471-03-4
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with pyridine;thionyl chloride in toluene at 110;
Steps:
24.1 Step 1
Pyridine (1.03 g, 12.97 mmol, 2 eq) and sulfoxide chloride (4.63 g, 36.91 mmol, 6 eq) were added to a toluene (30 mL) solution of compound 24a (1 g, 6.49 mmol, 1 eq); and after the addition was completed, the reaction solution was reacted for 3 hours at 110 °C. After the reaction was completed, the reaction solution was cooled to room temperature, added with water (40 mL), extracted with ethyl acetate (20 mL3); the organic phases were combined, washed with saturated brine (30 mL2), dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain crude compound 24b. 1H NMR (400 MHz, CDCl3): δ ppm 3.93 (t, J=6.8 Hz, 4H), 3.54 (t, J=6.8 Hz, 4H).
References:
EP4063371,2022,A1 Location in patent:Paragraph 0232-0234
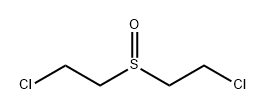
5819-08-9
3 suppliers
inquiry

471-03-4
4 suppliers
inquiry
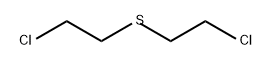
505-60-2
1 suppliers
inquiry

471-03-4
4 suppliers
inquiry