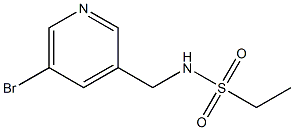
N-((5-bromopyridin-3-yl)methyl)ethanesulfonamide synthesis
- Product Name:N-((5-bromopyridin-3-yl)methyl)ethanesulfonamide
- CAS Number:1202552-53-1
- Molecular formula:C8H11BrN2O2S
- Molecular Weight:279.1541
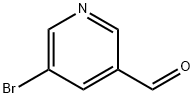
113118-81-3
277 suppliers
$5.00/250mg
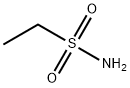
1520-70-3
124 suppliers
$21.00/1g

1202552-53-1
2 suppliers
inquiry
Yield:1202552-53-1 79%
Reaction Conditions:
Stage #1: 5-bromopyridine-3-carbaldehyde;Ethanesulfonamidewith titanium(IV) isopropylate in toluene at 115;
Stage #2: with methanol;sodium tetrahydroborate in dichloromethane at 0; for 0.5 h;
Steps:
Intermediate A- 11Ethanesulfonic acid (5-bromo-pyridin-3-ylmethyl)-amide
Intermediate A- 11Ethanesulfonic acid (5-bromo-pyridin-3-ylmethyl)-amideA flask was charged with 5-bromonicotinaldehyde (2.55 g, 13.7 mmol),ethanesulfonamide (2.99 g, 27.4 mmol) and toluene (250 mL), then titanium isopropoxide (5.84 g, 20.6 mmol) was added dropwise. The reaction mixture was heated to 115 °C over night and then concentrated in vacuo. The residue was taken up in DCM (200 mL) and MeOH (200 mL) and NaBH4 (1.04 g, 27.4 mmol) was added portionwise at 0 °C. The reaction mixture was stirred at 0 °C for 30 min and then poured into water (100 mL) and the resulting suspension was filtered through a pad of Dicalite and washed with DCM (3 x 100 mL). The aqueous layer was separated and extracted with DCM (2 x 200 mL).Combined organics were dried over Na2S04, filtered and preadsorbed on silica gel. The residue was purified by silica gel flash chromatography eluting with a 0 to 5% MeOH- DCM gradient to give the title compound (3.01 g, 79%) as an orange solid. MS: 279.0, 281.0 (M+H+).
References:
WO2013/37779,2013,A1 Location in patent:Page/Page column 84